THE VALORIZATION OF THE INTERMEDIATES IN THE PROCESS OF SUGAR BEET AS THE ALTERNATIVE RAW MATERIALS FOR THE BIOETHANOL PRODUCTION
DOI:
UDK:
JOURNAL No:
Volume 35, Issue 2
PAGES
71-76
KEYWORDS
bioethanol, fermentation, sugar beet, thin juice, thick juice, molasses
Rada Jevtić-Mučibabić1*, Jelena Dodić2, Jovana Ranković2, Siniša Dodić2, Stevan Popov2, Zoltan Zavargo2
1Institute for Food Technology, Novi Sad, Serbia
2 Faculty of Technology, University of Novi Sad, Serbia
ABSTRACT
Abstract
The aims of this study were to investigate the bioethanol production of thin and thick juice as intermediates from sugar beet processing in batch culture by free Saccharomyces cerevisiae cells and the effect of sugar concentration on ethanol yield. Thick juice and molasses of sugar beet from a domestic sugar factory were diluted with distilled water to give a total sugar concentration of 5, 10, 15, 20 and 25% (ww-1). Thin juice was diluted with distilled water to give a total sugar concentration of 5, 10 and 15% (ww-1). Initial concentration of fermentable sugars of 20% (ww-1) in culture medium can be taken as optimal, enabling maximal ethanol yield (68%). The efficiency of thin juice fermentation has higher values compared to the results received after the use of molasses for all three initial concentrations of the fermentable sugar concentration. The optimal concentration of fermentable sugar from thick juice for bioethanol production by free Saccharomyces cerevisiae cells was 20% (ww-1) at 30 °C, pH 5 and agitation rate 200 rpm gave maximum ethanol concentration of 12% (vv-1).
INTRODUCTION
The greatest challenges for the society are to meet the growing demand for energy of transportation, heating and industrial processes, and to provide raw material for the industry in a sustainable way. More importantly, the future energy supply must be met with a simultaneous substantial reduction of green house gas emissions. Actions towards this aim have been initiated. The European Commission plans to substitute progressively 20% of conventional fossil fuels with alternative fuels in the transport sector by 2020, with an intermittent goal set at 5.75% in 2010 (Gray et al., 2006; Schweitzer, 2006).
Liquid biofuels from renewable resources, particularly from lignocellulose materials, will have a substantial role in meeting goals (Herrera, 2006). Worldwide production in 2003 was approximately 30000 million litres (Fulton, 2004), dwarfing the output of potable ethanol – approximately 4000 million litres. The enalargement of the EU by countries of Central and Eastern Europe provide some more opportunities for biodiesel and bioethanol production, as those countries have presently double the acreage per citizen compared to the EU – 15 and have a significant potential in agroproductivity (Ericsson et al., 2006; Reiche, 2006). We expect biethanol to be one of the dominating renewable biofuels in the transport sector within the coming 20 years.
The varied raw materials used in the production of ethanol via fermentation are conveniently classified into three main types of raw materials: sugars, starches and cellulose. Sugars (from sugarcane, sugar beets, sweetsorghum, molasses and fruits) can be converted into ethanol directly. Starches (from corn, cassava, potatoes and root crops) must firstly be hydrolized to fermentable sugars by the action of enzymes from malt or molds. Cellulose (from wood, agricultural residues, waste sulfite liquor from pulp and paper mills) must likewise be converted into sugars, generally by the action of mineral acids (Walter et al., 2008). Molasses and other beet extracts do not require such treatment as the sugar content is almost all in the form of sucrose (Leiper et al., 2006). This is readily split into glucose and fructose in the initial stage of fermentation by the enzyme invertase, located in the periplasmic space between the yeast cell wall and cell membrane. The only preparation required with molasses is dilution to a suitable original gravity and pH buffering. From an economic point of view and in comparision with cereals, sugar beet and intermediates from beet processing are very good raw meterials for ethanol production due to their content of fermentable sugars, which can be directly used for fermentation without any modification.
Disadvantage of direct sugar beet and sugar beet pulp fermentation is a slow release of sugars from pulp into the fermented solution. The second aspect is a sort of problematic storability of beet that brings about sugar loss due to enzyme action (Hinkova et al., 2001). Raw juice contains about 15 – 20% of dry solids. Raw juice purity ranges between 85 and 90% that means there are about 85 – 90% of sugars and 10 – 15% of nonsugars in dry matter. Considering these facts, raw juice can be used straightaway after pH adjustment for fermentation (Hinkova et al., 2000). All these properties together with a relatively low price in comparison with other intermediates from beet processing make the raw juice a very profitable material for ethanol production. Its only disadvantage is low storability and casy decomposition by the action of micro-organisms. Thin juice is very suitable for ethanol production but the biggest disadvantage is a very small or hardly any possibility of its storage because the concentration of sugars is almost ideal for microbial growth. Thick juice is a relatively pure and highly concentrated sugar solution that is obtained by the concentration and thickening of thin juice on evaporators. This eliminates problems with storability that is comparable with molasses.
The aims of this study were to investigate of bioethanol production of thin and thick juice as intermediate from sugar beet processing in batch culture by free Saccharomyces cerevisiae cells and the effect of sugar concentration on ethanol yield and CO2 weight loss rate.
MATERIALS AND METHODS
2.1. Microorganism and substrate
Fresh commercial bakers yeast Saccha-romyces cerevisiae (Altech, Senta, Serbia) was used throughout this investigation. Thick juice and molasses of sugar beet from a domestic sugar factory were diluted with distilled water to give a total sugar concentration of 5, 10, 15, 20 and 25% (ww-1). The thin juice concentrations of 5, 10 and 15% (ww-1) were examined. The substrats were adjusted to pH 5.0 with 10% sulphuric acid (vv-1).
2.2. Fermentation
The fermentations were carried out in a 2.0 L laboratory bioraector with the fermentation medium of 1.5 L. The laboratory bio-reactor with the substrate was sterilized at 121 °C for 15 min. The addition of fresh commercial bakers yeast Saccharomyces cerevisiae (30% db) was 10 g per 1000 ml of medium to give a final concentration of 1 x 108 cells ml-1. Fermentation ran under anaerobic conditions for 72 h at the temperature of 30 °C and agitation rate 200 rpm.
2.3. Analiytical methods
During fermentation, the samples of the fermentation medium were taked in every four hour.
The fermentable sugar content (sucrose, glucose and fructose) of the supernate was determined by HPLC (HP 1090 Liquid Chromatograph, Hewlett Packard; column Zorbax Carbohydrate Analysis, 4.6x250 mm ID (5µm) with precolumn, Agilent; detector-1037A Refractive Index Detector, sensitivity 4xRIU; mobile phase 70:3 CH3CN/H2O (ww-1) at a flow rate of 1.0 mlmin-1; temperature column and detector 350C; injection volume 10 µL, manual).
The amount of soluble ash was calculated from the formula (Reinefeld et al., 1978; Herlich, 1990):
Ash (% db) = 0,0018 [a – (b x 0,9)] x 20(1)where: a – conductivity of sample (µS/cm)
b – conductivity of distilled water (µS/cm)
b – conductivity of distilled water (µS/cm)
Ethanol is determined by GC with a FID detector (Hewlett Packard 5890 Series II, Stockport, England). The ethanol yield (%) and fermentation efficiency (%) were expressed as ethanol % vv-1 per 100 g fermentable sugar utilized and % ww-1 fermentable sugar utilized per 100 g initial fermentable sugar, respectively.
2.4. Statistical analysis
All samples were prepared and analyzed in triplicates. The results were statistically tested by analysis of variance and the means were compared by the Scheffe test at a significance level of p=0.05, using the STATSOFT software (StatSoft, Inc., 1995).
RESULTS AND DISCUSSION
The composition of substrates (thin juice, thick juice and molasses) before dilution and fermentation were presented in Table 1.
Table 1. Composition of substrates thin juice, thick juice and molasses
Component |
(% w/w) |
|
||
|
|
thin juice |
thick juice |
molasses |
|
|
Sucrose |
13.13 |
57.85 |
49.30 |
|
|
Reducing sugars |
0.02 |
0.39 |
0.83 |
|
|
Fermentable sugars |
13.85 |
58.24 |
50.13 |
|
|
Free amino nitrogen |
0.13 |
0.18 |
1.87 |
|
|
Ash |
0.34 |
2.28 |
9.78 |
|
Results from Table 1 show that compo-sitions of thin juice, thick juice and of mo-lasses were characteristic and usual for sugar beet processing in domestic facto-ries. With their compositions, the given by-product, as well as the intermediate product are to be considered as convenient raw materials for cultivation media preparation for bioethanol manufacturing process.
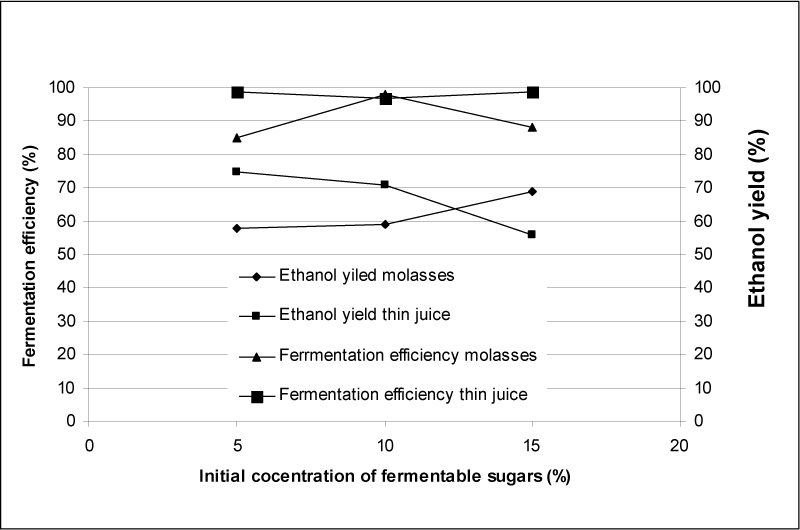 Figure 1. Effects of the initial concentrations of fermentable sugars from molasses and thin juice on ethanol yields and fermentation efficiency
Figure 1. Effects of the initial concentrations of fermentable sugars from molasses and thin juice on ethanol yields and fermentation efficiency
 Figure 2. Effect of the initial concentrations of fermentable sugars from molasses on the ethanol concentration during fermentation
Figure 2. Effect of the initial concentrations of fermentable sugars from molasses on the ethanol concentration during fermentation
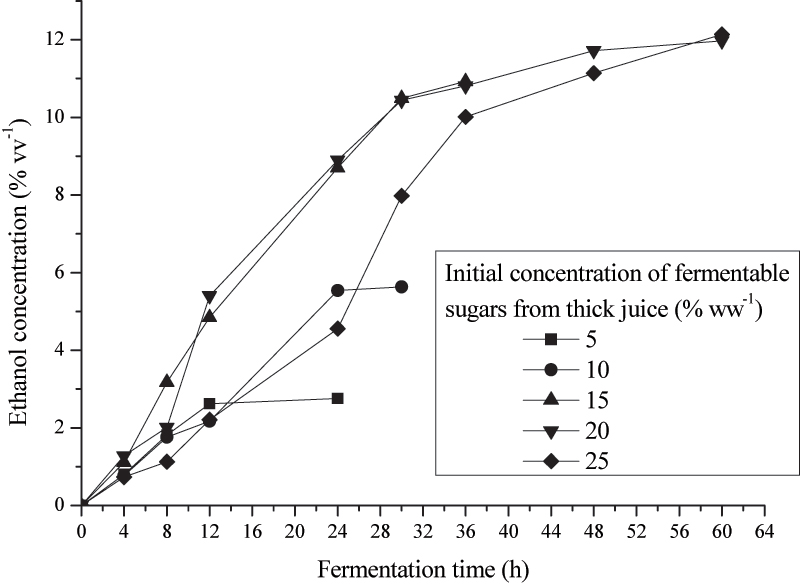 Figure 3. Effect of the initial concentrations of fermentable sugars from thick juice on the ethanol concentration during fermentation
Figure 3. Effect of the initial concentrations of fermentable sugars from thick juice on the ethanol concentration during fermentation
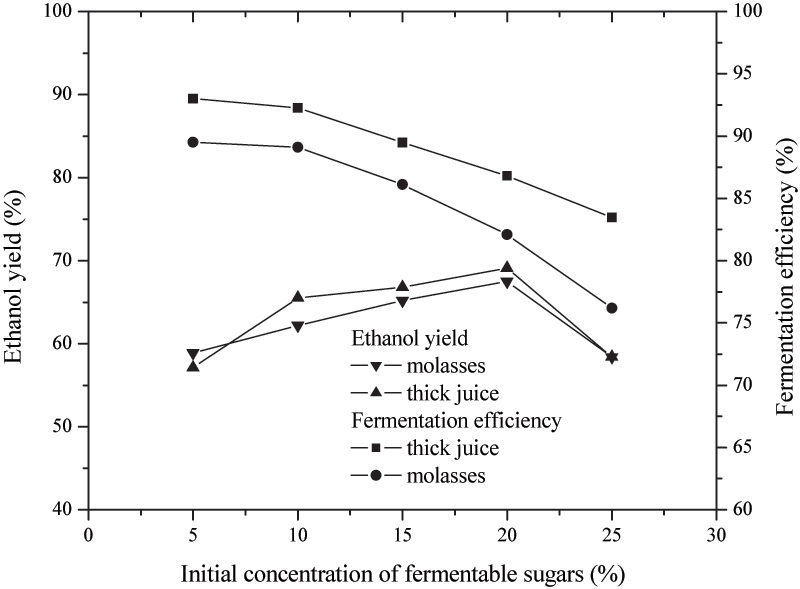 Figure 4. Effects of the initial concentrations of fermentable sugars from molasses and thick juice on ethanol yields and fermentation efficiency
Figure 4. Effects of the initial concentrations of fermentable sugars from molasses and thick juice on ethanol yields and fermentation efficiency
3.1. Ethanol yield and fermentation efficiency
Theoretically, the yield is 0.51l for ethanol and 0.489 for CO2 on a mass basic of glucose metabolized. In the industry, the ethanol yield that is calculated based on the total sugar feeding into the fermentation system without deduction of the residual sugar can be as high as 90 – 93% of its theoretical value of ethanol to glucose (Bai et al., 2008). Therefore, the residual sugar must be controlled for the residual reducing sugar and total sugar, respectively, in the ethanol production from starch materials. Any ethanol fermentation research which is expected to be practical needs to bear these criteria.
In the picture 1 there can be seen the dependancy of the fermentation efficiency, which presents the measure of the fermentable sugar usage, from the initial concentration of the fermentable sugars originating from molasses and thin juice. Based on the results, it can be noted that the efficiency of the fermentation from the molasses is the highest when applying the initial concentration of fermatable sugars of 10% (ww-1), whereas when applying the initial concentrations of 5 and 15% (ww-1) sugars the efficiency has somewhat lower value. The efficiency of the thin juice fermentatation has higher values compared to those received after the use of molasses and it shows the same results for all three applied initial fermatible sugar concentrations.
The picture 1 also shows the dependancy of the efficiency of ethanol yield, which presents the ratio of the ethanol content in the profermentable base and the quantity of the used fermentable sugars originating from fermentable sugars, from the initial position of the fermatable sugars originating from molasses and thin juice. Based on these results, it can be noted that the efficiency of the fermentation from the molasses is the highest when applying the initial concentration of fermatable sugars of 15% (ww-1), whereas when applying the initial concentrations of 5 and 10% (ww-1) sugars the efficiency has somewhat lower value.
 Figure 1. Effects of the initial concentrations of fermentable sugars from molasses and thin juice on ethanol yields and fermentation efficiency
Figure 1. Effects of the initial concentrations of fermentable sugars from molasses and thin juice on ethanol yields and fermentation efficiency Figure 2. Effect of the initial concentrations of fermentable sugars from molasses on the ethanol concentration during fermentation
Figure 2. Effect of the initial concentrations of fermentable sugars from molasses on the ethanol concentration during fermentationFigure 2 shows dependence of ethanol concentrations in fermenting mashes from fermentation times for different starting concentrations of fermentable sugars originating from molasses. It is possible to conclude that ethanol content in fermenting mashes during the very first 8 hours of fermentation, with all applied starting sugar contents, have almost linear growth. In further fermentation time, ethanol contents are mildly stagnant, and during a few last fermentation hours they are almost constant. With increasing of starting fermentable sugars concentrations in culture media, ethanol contents if fermenting mashes for each sampling time were significantly increased (p=0.022), but the necessary fermentation times were significantly increased (p=0.031). The results show, that sugar content of 20% (ww-1) in initial medium enables achieving of satisfactory ethanol contents in optimal duration of fermentation of 48 hours.
 Figure 3. Effect of the initial concentrations of fermentable sugars from thick juice on the ethanol concentration during fermentation
Figure 3. Effect of the initial concentrations of fermentable sugars from thick juice on the ethanol concentration during fermentationFigure 3 gives dependence of ethanol contents in fermenting mashes from duration of fermentation for different initial concentrations of fermentable sugars from thick juice. Results indicate that ethanol contents for all initial concentrations of fermentable sugars increase linear during first 12 hours of fermentation. With initial fermentable sugars concentrations of 15, 20 and 25% (ww-1) linear growth of ethanol contents prolongs to 28 hours. With further prolongation of fermentation times, ethanol contents in fermentation media stagnate.
With initial concentrations of fermentable sugars of 20 and 25% (ww-1), ethanol contents during the very last fermentation day were not significantly changed (p=0.072). After 24 hours of fermentationethanol contents reach nearly maximal values for 15, 20 and 25% (ww-1), of fermentable sugars, and as optimal concentration of fermentable sugars can be chosen the concentration of 20% (ww-1).
Figure 4 shows the dependences of ethanol yields (%) and fermentation efficiency (%) from initial concentrations of fermentable sugars in culture media with molasses or with the thick juice. The obtained results indicate that the with increasing of fermentable sugars content from 5 to 20% (ww-1), ethanol yields increase for both investigated raw materials (molasses and thick juice). When initial sugar content of 20% (ww-1) was increased to 25% (ww-1), the yields dropped significantly (p=0.021) from 67 to 56%. Initial concentration of fermentable sugars of 20% (ww-1) in culture medium can be taken as optimal, enabling maximal ethanol yield under cited experimental conditions. Efficiency of fermentation as a measure of spending of fermentable sugars was maximal at initial concentration of fermentable sugars of 5% (ww-1), and with the increasing of initial concentration of fermentable sugars it slowly falls down. Fermentation efficiencies of culture media with the thick juice were significantly higher (p=0.038) than those with molasses, at all concentrations of fermentable sugars.
 Figure 4. Effects of the initial concentrations of fermentable sugars from molasses and thick juice on ethanol yields and fermentation efficiency
Figure 4. Effects of the initial concentrations of fermentable sugars from molasses and thick juice on ethanol yields and fermentation efficiencyCONCLUSION
Bioethanol production from sugar beet by free Saccharomyces cerevisiae cells technology is promising as an alternative fuel. This work has confirmed that the production of bioethanol from thick juice as intermediate of sugar beet processing is technically possible. This gives the benefits of reduced water usage, reduced waste water purification costs, easier mixing with syrup if used warm, lower use of acids for pH buffering, and increased levels of nutrients. The efficiency of thin juice fermentation has higher values compared to the results received after the use of molasses for all three initial concentrations of the fermentable sugar concentration. The optimal concentration of fermentable sugar from thick juice for bioethanol production by free Saccharomyces cerevisiae cells was 20% (ww-1) at 30 °C, pH 5.0 and agitation rate 200 rpm gave maximum ethanol concentration of 12% (vv-1).

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE