INVESTIGATION OF THE BASIC PARAMETERS OF THE COAGULATION AND FLOCCULATION PROCESS INFLUENCED BY THE BEAN SEED EXTRACT
Uroš D. Miljić 1*, Jelena M. Prodanović 1, Marina B. Šćiban 1
1Faculty of Technology, University of Novi Sad, Bul. cara Lazara 1, 21000 Novi Sad, Serbia
ABSTRACT
Abstract
In this paper the coagulation properties of the bean seed extract, as the alternative means for removal of the turbidity of water was examined. The bean seed extract was prepared by extraction with the distilled water. Coagulation activity of the extract was the best when the extraction was performed in duration of 20 minutes at 20 ºC. The efficiency of so obtained clarifying agent was investigated with the artificial turbid water prepared with the addition of the kaolin slurry into the municipal tap water. Clarity of the water was measured with the haze meter. The coagulation activity was insignificantly better when Ca(OH)2 instead of NaOH was used as the means for the pH value adjusting of the model water. In conclusion, the coagulation with the beans extract, a process with the rapid mixing for 1 minute and slow mixing for 30 minutes, could be adopted for the removal of water turbidity.
INTRODUCTION
The compounds isolated from natural raw materials having the coagulation inducing activity can be considered as the natural coagulants. Natural coagulants are, most often, isolated from plants and their chemicals structures can be very diverse. The investigations have shown that the natural coagulants also can be obtained from microorganisms and from animal tissues, as well [Kawamura, 1991]. Raw materials of animal origin have not been investigated sufficiently yet, while the application of natural coagulants still lies in its initial phase, and additionally, the processes necessary for the processing of these materials are costly and complicated. For example microbiological processes create good protein yields but they must be accompanied with the additional purification steps, making such procedure more expensive compared to the one based on the raw materials of plan origin. The basic advantage of natural coagulants represents the fact that it leaves the biodegradable residue that can be easily processed and disposed [Ozacar et al., 2003]. A number of plant materials containing potentially the coagulation stimulating substances, such as bark and core of the horse chestnut, acorns, acacia, locust bean, common bean, soybean, etc. [Stojimirović, 2005; Homa, 2006; Beljkaš, 2007]. Until now, the best examined is extract obtained from seeds of Moringa oleifera, which showed the highest effects as the primary coagulant and which can be compared with aluminum sulphate, the most often used coagulant for purification of water [Berger et al., 1984]. Berger et al., [1984] concluded that active components from M. oleifera responsible for the clarification of water are proteins. At concentrations used for the water clarification, this coagulation agent is not dangerous in respect to the human health, quite opposite to the aluminium-containing compounds which are considered to induce the Alzheimer’sisease [Mallevialleet al.,1984].
MATERIALS AND METHODS
Artificial turbid water was applied as the model water for the determination of coagulation activity of the partially purified extract of proteins from the bean seeds. The model water was obtained by the dilution of 15 ml suspension of kaolin with the tap water immediately before the coagulation experiments, using the addition of 5 mL of the suspension per 1 L of water. So prepared model water had the turbidity of 35 NTU. Such model water was chosen because of the comparability with the data from the literature, while the experiments were mostly performed with model water turbidified with kaolin.
Preparation of bean seed extract
The preparation of natural coagulants has been done from the seed of white beans obtained from the Institute of field and vegetable crops of the Faculty of agronomy, Novi Sad. White bean seeds were grounded and sieved with the sieve with light area width of 0.4 mm, then suspended in the distilled water at the concentration of 10 g/L, with the intensive mixing with magnetic stirrer for 10 minutes in order to improve the extraction. So obtained suspension was filtered, using the widepore filter paper, in order to remove solid residues.
Analytical methods
Water turbidity testing, after addition of the coagulant and the finished settling, was performed by the nephelometric method, using the haze meter VOS 4000, according to the instruction of the manufacturer. The obtained values for turbidity were expressed in NTU units (nephelometric turbidity unit). pH values were determined by the pH-meter HI 9321.
Experimental procedure
Immediately before the experiment, the model water was prepared by the dilution of the kaolin suspension with the tap water. In the so obtained model water, on the magnetic stirrer, pH value was adjusted to the desired value, using the additions of the solutions of NaOH (1 Mol/L) or HCl (1 mol/L). Model water was divided into four beakers, 300 mL per beaker, which were introduced into the mashing bath Glasbläserei (water bath with the mixer) and mixed with the higher mixing speed (200 rpm) for 1 or 2 minutes. At the very beginning of fast mixing, in glasses 0.9 mL of the coagulant suspension was added, corresponding to the extract from 30 mg of beans per 1 L of the model water. After that, mixing rate was decreased to 80 rpm. Slow mixing duration was 15, 30 or 60 minutes.
After finishing the mixing, metal beakers were taken out from the apparatus; their contents were transferred into glass beakers, where they were left for 1 hour in order to settle. The blank assay was the model water that was also poured into the beaker, and left to settle for 1 hour. After that, 100 mL of the clarified supernatants (upper layer) were pipetted off and their turbidities were measured. The coagulation activity was calculated with the application of the following formula
where: CA = coagulation activity; Tba = turbidity of the blank assay; Ts = turbidity of a sample.
RESULTS AND DISCUSSION
The effects of the pH value on the coagu-lation with the bean seeds extract
Colloid particles that induce the turbidity are so hydrated that their density approximately equals to the density of water. Owing to that, their removal by sedimentation is very slow or impossible. Besides that, the existence of charge on the particles prevents them joining. At the isoelectric point the particles are electrically neutral, so they join with each other and settle to the bottom. With increasing distance of the pH value from the isoelectric point, the organic substance suspensions are more stable.
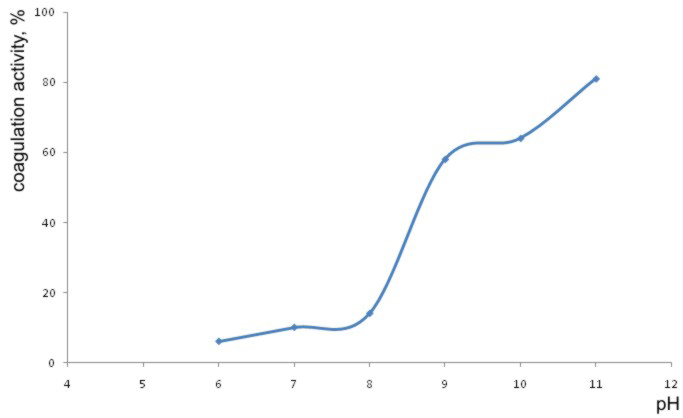
Having this in mind, the coagulation activities of extract of bean seeds were determined at pH values between pH = 6 and pH = 11. For these experiments, 3 mL of extracts per 1 L of water were used. The results are shown in the Graph 1.
As the results outlined in the Graph 1. show, the coagulation activity quickly increases at the pH values higher than pH 6,0. Similar results were obtained with the M. oleifera extracts [Okuda et al., 2001]. In further experiments pH values were adjusted to pH = 9.
The effects of NaOH and Ca(OH)2 as agents for the pH values adjustment for the coagulation with bean seeds extracts
In this experiment, the influence of agents for adjusting of the pH values of model water on the coagulation process was examined.
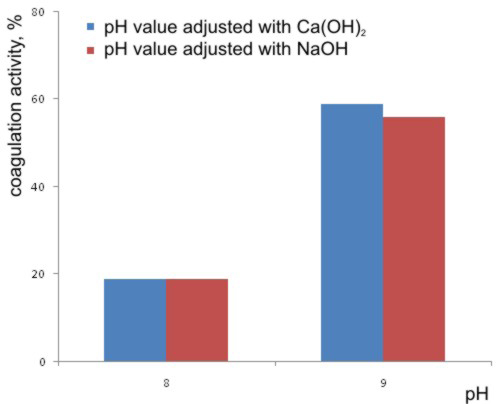
For these purposes, the solutions of NaOH or Ca(OH)2 were used, and the pH values were adjusted to pH 8 or to pH 9. The results are shown in the Graph 2.
It can be concluded that the pH value adjusting with the Ca(OH)2 at pH 9 gives slightly better results than the NaOH solution with respect to the coagulation activity. The reason is that the Ca(OH)2 solution brings into water the twovalence ions, so that the coagulation is improved owing to the formation of net structures with the coagulants, which improves the formation of floccules [Okuda et al., 2001]. Besides to that, Ca(OH)2 is less expensive than the NaOH which is additional advantage. But, considering the instability of the Ca(OH)2 suspension, in the rest of laboratory experiments, nevertheless, the NaOH solution was used for adjusting the pH values in the model water.
Investigation of the effects of extraction time and temperature of extraction on the coagulation activity of the bean seed extract
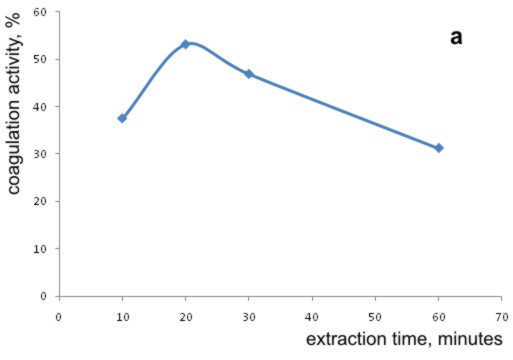
In order to achieve the best possible extraction of the coagulation inducing substances from the bean seeds, investigations of the extraction times were performed. The extractions lasted 10, 20, 30 and 60 minutes at the ambient temperature and the same rates of mixing. The obtained extracts were examined in an experiment of coagulation of the model water with the turbidity of 35 NTU, and the pH value of 9. The effect of the bean seed extraction time in these experiments is outlined in Graph 3a.
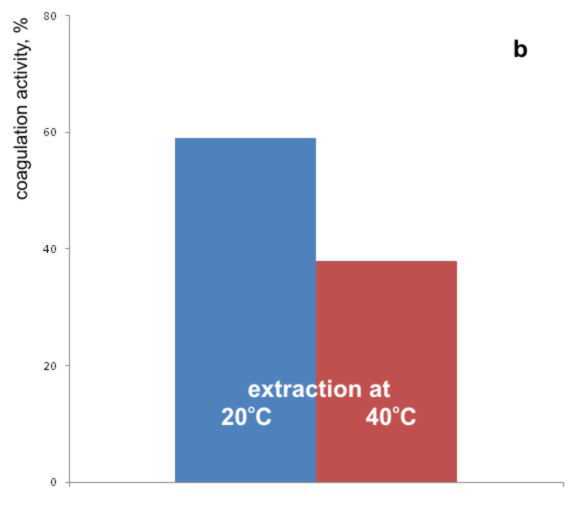
Graph 3. Effects of (a) the extraction time, and (b) temperature of extraction on the coagulation activity of bean seed extract
From the Graph analysis it is possible to conclude that the process of coagulation is more efficient if extracts obtained after extraction of active components lasting more than 10 minutes were used. With the extraction time of 20 minutes coagulation activity was maximal, and with further increasing of the extraction time (30 and 60 minutes), coagulation activities of extracts were decreased. For comparison, extraction time of the active coagulation-stimulating components from the M. oleifera seeds was 10 minutes [Okuda et al., 1999].
In the second part of these experiments, extracts were prepared according to the standard procedure, but the extraction in one case was performed at the ambient temperature of 20 0C (standard procedure) and in the other at 40ºC. The obtained results are shown in the Graph 3b.
From the Graph 3b it can be concluded that the better removal of turbidity was achieved with extracts obtained at 20ºC. It can be supposed that at 40 ºC a thermal inactivation of a part of active components from bean seed extract occurs, because the active component is supposedly of the proteinaceous nature [Ndabigengesere et al., 1995].
Investigation of the effects of mixing time on the coagulation activity of bean seed extract
In order to find out the best possible conditions for the interaction of the coagulant and the model water, investigation of modifications in the standard procedure of the colloid activity was performed, experimenting with duration of mixing athigher and lower rates.
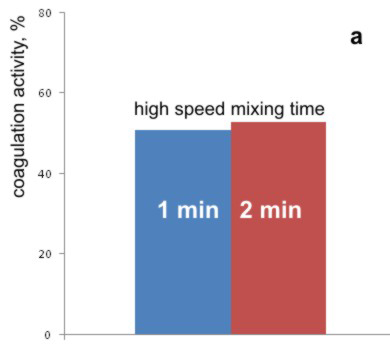
In the first part of these investigations, the influence of time period of mixing with high rate of the bean seeds extract and model water was examined. To the model water with the turbidity of 35 NTU with pH value adjustted to 9, the extract of bean seeds was added, and, after fast mixing times of 1 or 2 minutes, slow mixing was continued for 30 minutes. The realized colloid activity is shown on the Graph 4.a.
Analysis of the Graph 4.a shows that the increasing of period of mixing with the high rate to 2 minutes does not exhibit any significant effect on the coagulation activity, compared with the standard procedure, where the mixing lasted for 1 minute.
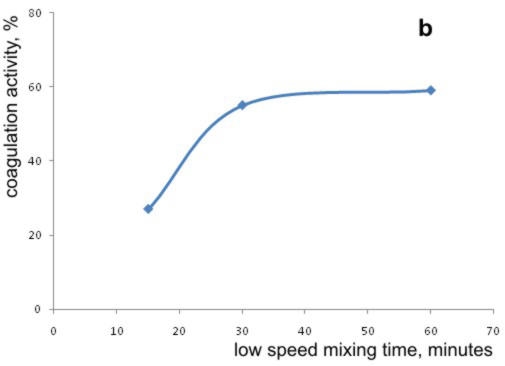
Graph 4. Effects of (a) high speed, and (b) the low speed mixing times on the coagulation activity of bean seed extract
In the second part of the experiment, the influence of duration of slow mixing period of the bean seed extract with the model water was examined. To the model water with the turbidity of 35 NTU, with the pH value adjustted to pH 9, the bean seed extract was added and after the high speed mixing, periods of slow mixing lasting 15, 30 and 60 minutes were applied. The obtained results are shown in Graph 4.b.
As it can be observed, the slow mixing time of 15 minutes was not long enough to achieve the coagulation activity obtained with the standard procedure. Coagulation activity was somewhat better, when the slow mixing time was increased from the standard 30 minutes to 60 minutes. However, this increase was only 5.1%, so the slow mixing period of 30 minutes can be considered as acceptable and more economical.
CONCLUSION
On the basis of the performed experiments and obtained results for the investigation of colloid activity of bean seed extract, the following conclusion can be drawn:
For a good coagulation activity the pH value of the processing water has to be adjusted to pH 9. Better results are obtained when the pH value was adjusted with the Ca(OH)2, instead of NaOH, which is convenient from the point if view of the economics, while the instability of Ca(OH)2 should not be underestimated.
In the experiments to determine the mixing time for bean seed extract coagulation activity, conditions including 1 minute of rapid mixing and 30 minutes of slow mixing can be considered optimal.

 JOURNAL TOOLS
JOURNAL TOOLS
 DOWNLOAD PDF
DOWNLOAD PDF
 INSTITUTE
INSTITUTE