ABSTRACT
Abstract
Regarding the tendency of food industry to use natural colorants, ground paprika and oleoresins have become especially attractive. However, carotenoids, the main coloring agents of paprika are prone to oxidative degradation. In order to achieve standard quality of the final products, there is a need to understand carotenoid composition and content change during the period of ripening, postharvest manipulation and technological process of ground paprika production.
Thus, the aim of this study was to determine carotenoid composition and content in fresh and dry pepper fruits. In order to achieve that, variety of Capsicum annuum “AlevaNK” intended for production of paprika powder in brown phase of maturity was used. Carotenoids were extracted from fresh and dry pepper samples with acetone and after purification, separation and determination of extracted carotenoids was performed by reverse phase HPLC system with DAD detection.
Referring to the obtained results, drying and milling of pepper fruits caused a decrease in number of free and esterified carotenods. Ripening, milling and drying processes resulted in decrease in number of identified carotenoids of paprika. Also, increase of proportion of mono and diesterified carotenoids, increase of proportion of red carotenoids and decrease of proportion of yellow ones and increase of proportion of capsanthin occurred during the postharvest treatment of paprika .
INTRODUCTION
The main quality parameters for ground paprika products are color and pungency (Carvajal et al., 1998; Govindarijan, 1986; Govindarijan et al., 1987). Color of ripe pepper fruits originates from carotenoids, and two red carotenoids, capsanthin and capsorubin can naturally be found only in Capsicum
Two main roles of carotenoids in photosyn-thetically active plant tissue are: to trap light energy at wavelengths scarcely accessible to chlorophyll and to protect photosynthetic apparatus, dissipating energy and “quenching” reactive harmful species such as singlet oxygen and excited chlorophyll (Frank and Cogdell, 1993). Additionally carotenoids in flowers, fruits and seeds have role to attract pollen- and seed- dispersion animals, as well as to dissuade potential pests (Goodwin, 1952). Examples of carotenoid rich fruits are peppers (Capsicum annumm), tomatoes (Lycopersicon esculentum) and oranges (Citrus aurantium) (Goodwin, 1976).
Ripening of red pepper fruits occurs gradually and it can be observed as color change from green to brown and further to red and finally deep red. These visible changes in fruit appearance are used by many researchers as a degree of fruit maturity and thus phases of development in which samples are taken (Hornero-Méndez and Mínguez-Mosquera, 2000; Deli et al., 2001). Visual phases of fruit development are also used in investigation of volatile compounds (Pino et al., 2006; Sousa et al., 2006)
Beneath visual color change of pepper fruit, intensive biosynthesis of carotenoids occurs which leads to increase of precursors: β-carotene, β-criptoxantin and zeaxanthin. These compounds are further transformed to antheraxantin and violaxanthin which are substrates for the capsanthin-capsorubin synthase, enzyme which activity produces capsanthin and capsorubin, red carotenoids which are exclusively present in Capsicum spp. genus (Hugueney et al., 1995; Hirschberg, 2001). These processes also take place after harvest and at the begging of drying process (while temperature of the air is relatively low and moisture level of fruit tissue high) of production of ground paprika spice (Hornero-Méndez and Mínguez-Mosquera, 2000). During maturation, parallel to biosynthesis and transformation, process of acylation of carotenoid by fatty acids occurs. This process leads to partially as well as completely esterified carotenoids (Mínguez-Mosquera and Hornero-Méndez, 1994; Breithaupt and Schwack, 2000).
Ripening of pepper fruits implies de novo carotenoid biosynthesis which can be seen as fruit color change. Characteristic color changes through which pepper fruits pass are: green, when chlorophyll is the main pigment, brown which is characterized intensive biosynthesis of carotenoids especially red ones and degradation of chlorophylls, red and deep red when red carotenoids dominate in quantity (Mínguez-Mosquera and Hornero-Méndez, 1994). In pepper fruits intended for ground paprika production intensive biosynthesis can increase total carotenoid content 66 fold (Deli et al., 2001). Also, during ripening esterification of carotenoids with fatty acid occurs and at the end of ripening period the majority of carotenoids are esterified (Breithaupt and Schwack, 2000).
As stated earlier, the main quality parameters for ground paprika products are color and pungency, while aroma recently has started to receive some attention (Mateo et al., 1997; Cremer and Eichner, 2000; Kocsis et al., 2002, 2003). Although main role of carotenoids in quality of ground paprika spice is color, carotenoids have very important role in aroma due to sensitivity of carotenoids to drying process in hot air. Independently of esterification degree, all carotenoids are vulnerable to heat. Especially sensitive to thermal degradation is distinctive part of all carotenoids – diene chain (Laffingwell, 2002). The common carotenoid degradation pathways are isomerisation, oxidation and fragmentation, (Bonnie and Cho, 1999). Unique properties of carotenoids are highly dependable of their structure, so any modification of the basic skeleton, such as cyclization, double bond migration, introduction of oxygen and shortening of diene chain through thermal or chemical reaction, can result in loss of their properties. Two types of products are formed during thermal degradation of carotenoids: volatile fraction of low molecular weight which is vaporized and nonvolatile fraction from larger fragments of the carotene molecules. Volatile compounds that originated from carotenoids can be found in many plants (Laffingwell, 2002).
Having in mind the tendency of food industry to use natural colorants, ground paprika and oleoresins have become especially attractive because of the possibility to standardize their quality e.g. coloring power. Aim of this study was to determine changes in carotenoid composition and content in pepper fruits before and after drying and milling.
MATERIALS AND METHODS
Plant material
Cultivar “AlevaNK” of pepper intended for paprika production was used in this study. AlevaNK was introduced in year 2000. The main property of this cultivar is a high yield and unified fruit ripening meaning that ¾ of fruits are collected in the first harvest and ¼ in the second harvest. Pepper fruits were sampled from 8 ha field located in Fruška gora mountain, near the town of Stara Pazova. Pepper fruits from selected field were intended for paprika production. Approximately 25 kg of the fruits was sampled from field in brown phase of maturity, during intensive biosynthesis of carotenoids and decomposition of chlorophyll. Fruits were divided into two lots. Fresh fruits from the first lot were used immediately for the sample preparation. Fruits from the second lot were left for two days for further ripening in one fruit layer in glasshouse under semicontrolled conditions, and then dried in a laboratory dryer with fitted fan at 60±5 °C. Dried fruits were milled in a coffee grinder and obtained powder was used for further analysis.
Sample preparation
Similar procedure for extraction of carotenoids was performed for both samples, fresh and dry. Peduncle and seed were removed and only pericarp was used for further analysis. Carotenoids present in fresh sample were extracted from 10 g of 0.5 X 0.5 cm representative pericarp cube cuttings, while for dry sample, pericarps from all fruits were milled in a coffee grinder and 1 g of obtained powder was used for extraction. Extraction was carried out with 50 mL portions of cold (4 °C) acetone using ultra turrax and magnetic stirrer for fresh and dry samples respectively. Extraction was repeated until the loss of color, which usually required 4 X 50 mL per sample. Obtained acetone extracts were combined in separation funnel and carotenoids were extracted with 100 mL of cold (4 °C) diethyl ether. A sufficient quantity of 10% NaCl was added to aid the separation of the phases. Ethyl ether phase containing extracted carotenoids was concentrated to the volume of 25 mL, using rotary vacuum evaporator. Obtained extracts were stored at -20 °C and filtered through 0.45 µm pore size PTFE filter (Rotilabo-Spritzenfilter 13 mm, Roth, Karlsruhe, Germany) before injection into the HPLC system.
Instrumentation
Chromatographic conditions for the carotenoid determination were performed according to the method of Morais et al. (2001) with certain modifications (Table 1).
Table 1. Configuration and operating conditions of the HPLC system
Instrument: |
Liquid Chromatograph HP1090, HewlettPackard
|
|
Detecor:
|
Diode Array Detector (DAD)
|
|
Wavelength:
|
460±4 nm
|
|
Injection volume:
|
10 µL, manually
|
|
Column:
|
Zorbax SB C18, 3.0×250 mm i.d., particles 5 μm
|
|
Precolumn:
|
Zorbax SB C18, 4.6×12 mm i.d., particles 5 μm
|
|
Flow rate:
|
1.5 mL/min
|
|
Mobile phase:
|
A: acetone-water (75:25, v/v);
B: acetone-methanol (75:25, v/v)
|
|
Gradient:
|
from 0 to 25% B in 10 min,
from 25 to 100% B in 35 min,
100% B in 45 min and
0% B in 65 min
|
|
Post time:
|
15 minutes
|
Capsorubin, antheraxanthin, zeaxanthin, violaxanthin and β-carotene standards were acquired from Carotenature (Switzerland), while capsanthin was purchased from Hoffman La Roche (Switzerland).
Carotenoids in the sample extracts were identified by matching the retention time and their spectral characteristics against those of the standards. Contents of pigments are expressed as the ratio of carotenoid peak area to the total peak area.
RESULTS AND DISCUSSION
Chromatogram of separated standards under the given conditions is shown in Figure 1. Standard carotenoid mix was successfully separated under the conditions given. All carotenoids were eluted in first 10 minutes, with the exception of β-carotene for which the retention time was 34.4 minutes.
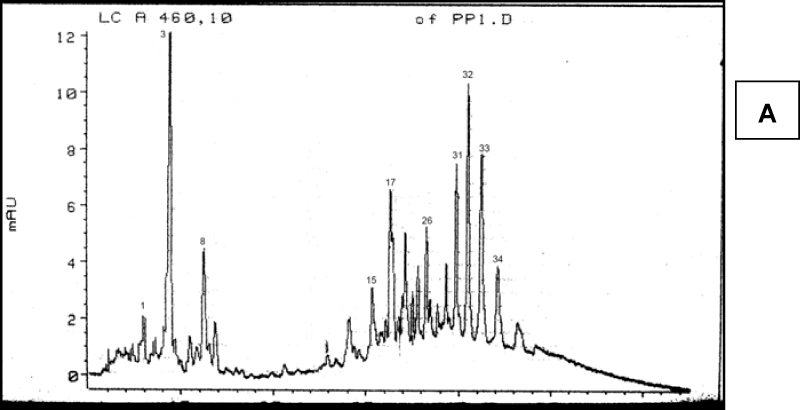
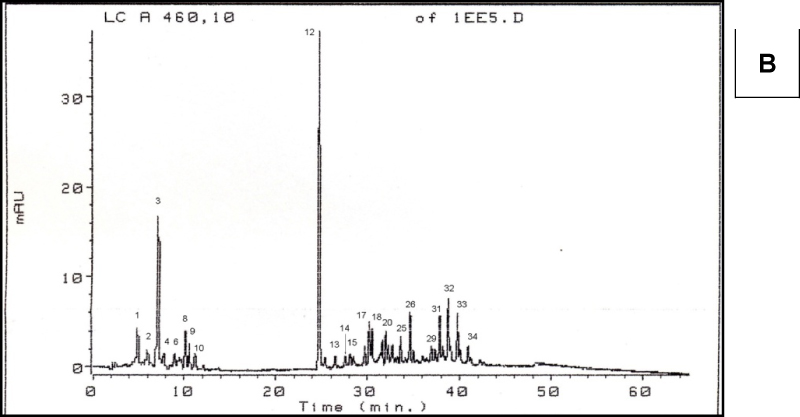
Chromatograms of fresh and dry pepper fruit samples in brown phase of maturity are shown in Figure 2. Based on retention time, carotenoids could be divided into three groups. The first part of chromatogram belongs to the majority of free carotenoids, which ends at peak 14. The second part of chromatogram belongs to monoesterified carotenoids and it starts with peak 15 and continues until β-carotene (peak 26). Peaks which belong to the third part of the chromatogram are diesterified carotenoids. Similar conclusion about the position of monesterified and diesterified carotenoids at chromatograms was made by Biacs et al. (1989). Concerning the esterification degree of carotenoids, capsorubin, violaxanthin, capsanthin, anteraxanthin and zeaxanthin were present in free form, capsanthin and capsorubin were present as mono and di- estirified forms while zeaxanthin was presented in diesterified form. Identification of peaks of the esterified carotenoids was based on the absorption spectra.
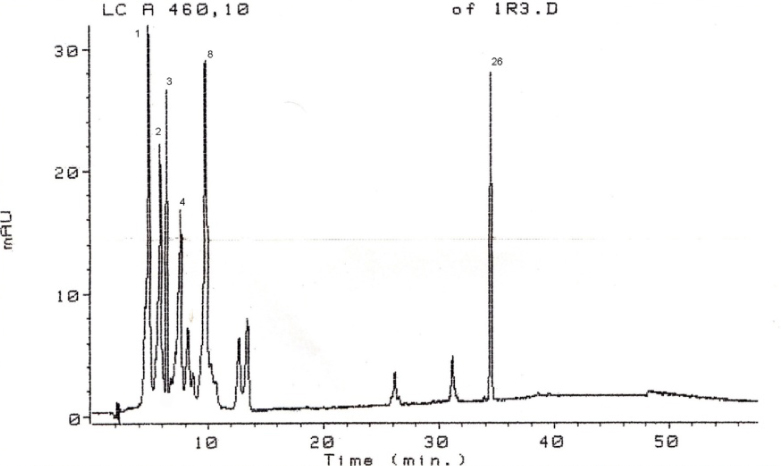
Figure 1.Separated carotenoid standards. 1-capsorubin, 2-violaxanthin, 3-capsanthin, 4-antheraxanthin, 8-zeaxanthin and 26-β-carotene
Figure 2. Chromatograms of carotenoids of brown pepper fruits. A – fresh, B – dry fruit. Peak numbers are presented in Table 1.
Table 2. Content (%) of identified carotenoids in fresh and dry brown fruits. Max. abs. – maximums of absorptions on absorption spectra, tR – compound retention time. Compounds from 1-14 – free carotenoids, 15-25 – monoesterified cartenoids, 26 and 27 non-esterified β-carotene and cis-β-carotene and from 28-34 diesterified carotenoids.
Peak No. |
Compound
|
Fresh fruit (%)
|
Dry fruit (%)
|
Max. abs. (nm)
|
tR(min)
|
|
1
|
Capsorubin
|
3.8
|
2.3
|
446,470,480
|
4.883
|
|
|
2
|
Violaxanthin
|
3.0
|
|
421,442,473
|
5.788
|
|
|
3
|
Capsanthin
|
17.4
|
20.2
|
(446),470,486
|
6.928
|
|
|
4
|
Anteraxanthin
|
1.8
|
|
422,445,472
|
7.501
|
|
|
6
|
n.i.
|
1.5
|
|
(402),432,454,488
|
8.678
|
|
|
7
|
n.i.
|
2.0
|
|
(UV),___,464-2,486
|
9.246
|
|
|
8
|
Zeaxanthin
|
3.4
|
8.0
|
430,452,479
|
9.895
|
|
|
9
|
n.i.
|
2.4
|
|
(430),448,475
|
10.665
|
|
|
10
|
n.i.
|
1.6
|
|
(430),448,475
|
11.740
|
|
|
12
|
n.i.
|
21.2
|
|
|
22.220
|
|
|
13
|
n.i.
|
0.5
|
|
(434),452,476
|
24.633
|
|
|
14
|
n.i.
|
0.5
|
1.6
|
(434),452,476
|
26.177
|
|
|
15
|
Capsorubin
|
1.1
|
3.2
|
(446),470,480
|
27.802
|
|
|
16
|
Capsanthin
|
1.4
|
|
(446),470,486
|
29.450
|
|
|
17
|
Capsanthin
|
4.0
|
11.3
|
___,474,(502)
|
29.915
|
|
|
18
|
Capsanthin
|
2.4
|
|
___,474,(502)
|
30.244
|
|
|
20
|
Capsanthin
|
2.4
|
1.8
|
(446),470,502
|
31.5
|
|
|
21
|
Capsanthin
|
1.0
|
|
___,468,___
|
B 31.850 |
|
|
23
|
n.i.
|
0.2
|
|
(UV),(408),430,454
|
32.768
|
|
|
24
|
n.i.
|
0.5
|
|
(430),456,___
|
33.041
|
|
|
25
|
n.i.
|
1.7
|
2.7
|
(428),450,475
|
33.384
|
|
|
26
|
b-carotene
|
3.0
|
3.9
|
(428),450,484
|
34.442
|
|
|
27
|
cis-b-carotene
|
0.7
|
1.6
|
(352,366),(422),450,484
|
34.794
|
|
|
28
|
Capsorubin
|
0.5
|
|
UV, ___,460,480
|
35.832
|
|
|
29
|
Capsanthin
|
2.3
|
2.9
|
(444),474,502
|
36.760
|
|
|
30
|
Capsorubin
|
1.2
|
|
UV, 434,454,484
|
37.140
|
|
|
31
|
Capsanthin
|
4.0
|
7.4
|
___,472,(500)
|
37.677
|
|
|
32
|
Capsanthin
|
5.1
|
13.6
|
___,472,(500)
|
38.551
|
|
|
32a
|
Zeaxanthin
|
1.6
|
|
(358),___,460,480
|
38.762
|
|
|
33
|
Capsanthin
|
4.0
|
12.5
|
___,472,(500)
|
39.536
|
|
|
33a
|
Zeaxanthin
|
1.8
|
|
(358),___,460,480
|
39.792
|
|
|
34
|
Capsanthin
|
1.8
|
6.8
|
___,472,(500)
|
40.664
|
|
n.i. - not identified carotenoids
As indicated by the resuls shownt in Table 2, drying and milling caused a decrease in the number of present carotenoids in the investtigated samples. Nonesterified carotenoids were the most affected, since only 6 of 14 carotenoids (including β-carotene and cis-β-ca-rotene) were identified after drying and mi-lling. Due to their chemical properties, caro-tenoids are sensitive to oxidation that can cause changes to double bounds of long chain of conjugated dienes. By using mixture of 6 carotenoid standards, approx. 68% of to-tal peak area wes identified in fresh and 95.7% in dry brown pepper fruit.
Carotenoids found in red capsicum fruits can be divided, by visual appearance, to red and yellow ones. Red carotenoids presented in Table 2 are capsanthin and capsorubin and their esters. Biosynthesis and degradation of carotenoids during ripening and drying could also effect the content of red and yellow carotenoids. Content of red and yellow caro-tenoid and the ratio of capsanthin and cap-sorubin is shown in Table 3.
Table 3. Content (%) of total red, yellow carotenoids and capsanthin and capsorubin in fesh and dry brown paprika
|
|
Fresh (%)
|
Dry (%)
|
|
Total yellow
|
44.0
|
17.9
|
|
Total red
|
56.0
|
82.1
|
|
Capsanthin
|
49.3
|
76.6
|
|
Capsorubin
|
6.7
|
5.5
|
Results from Table 3 show that drying and milling process decreased the content of yellow pigments and increased the content of total red carotenoids as well as the content of capsanthin. Process of milling, which results in increase of the area of pericarp exposed to oxygen from air and heat used for drying and generated from milling process is certainly one of the reasons of degradation of caro-tenoids (Minguez-Mosquera et al., 1994; Márkus et al., 1999; Minguez-Mosquera et al., 2000).
CONCLUSION
The described sample preparation and HPLC method on Zorbax SB C18 column enables a successful separation of carotenods present in fresh and dry brown paprika. In total, 32 peaks were identified as free, mono and di- esterified carotenoids. Dominant pigment in brown pepper was capsanthin, as it is in red peppers.
Ripening, milling and drying processes resulted in decrease in number of identified carotenoids in paprika, in the share increase of mono and diesterified carotenoids, share increase of red carotenoids and share decrease of yellow ones and share increase of capsanthin.
Download full article PDF
 DOWNLOAD PDF
DOWNLOAD PDF
 Figure 1.Separated carotenoid standards. 1-capsorubin, 2-violaxanthin, 3-capsanthin, 4-antheraxanthin, 8-zeaxanthin and 26-β-carotene
Figure 1.Separated carotenoid standards. 1-capsorubin, 2-violaxanthin, 3-capsanthin, 4-antheraxanthin, 8-zeaxanthin and 26-β-carotene
 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE