Influence of growth conditions on biofilm formation of Listeria monocytogenes
ABSTRACT
INTRODUCTION
Listeria monocytogenes is a ubiquitous gram-positive facultative intracellular bacterial pathogen which provokes listeriosis, a severe disease with high hospitalization and mortality rates, with the consumption of contaminated food being the principle mode of its transmission to humans. Due to the ubiquitous nature and hardy growth characteristics of this bacterium, L. monocytogenes is able to contaminate and thrive in the food-processing environment (Kadam et al., 2013). In particular, the psychrotrophic nature of L. monocytogenes allows replication in refrigerated, ready-to-eat food products that have been contaminated during processing and packaging. Consequently, L. monocytogenes is frequently associated with food-borne disease outbreaks that are characterized by widespread distribution and relatively high mortality rates (Donlan and Costerton, 2002; Donnelly, 2001).
This organism possesses the ability to adhere to and colonize on abiotic surfaces to form biofilms (Yousef and Carlstrom, 2003). Once bacteria adhere to solid surfaces and form biofilms, they became more resistant to cleaning and sanitation treatment, and cells detaching from the biofim can further turn into the source of persistent contamination (Hood and Zottola, 1997; Donlan and Costerton, 2002). In the food industries, biofilm accumulations have been found on walls, floors, drains, rubber, conveyor belts, processing equipment, which is more resistant to disinfectants and sanitizing agents than planktonic cells (Milovanov et al., 2015; Moltz and Martin, 2005).
The factors governing the biofilm formation of bacteria to surfaces are still not well understood. Therefore, the aim of this study was to determine the biofilm forming behavior of nine L. monocytogenes strains in two different media such as Tryptone soya yeast extract broth (TSYEB) or Brain-heart infusion broth (BHI) at temperatures 7 ºC, 25 ºC , 37 ºC, 42 ºC during 5 days.
MATERIAL AND METHODS
Strains and growth conditions
A total of 9 L. monocytogenes strains were used in this study. Tests were performed with 8 isolates of L. monocytogenes (Lm1-Lm8) isolated from meet processing industry. L. monocytogenes ATCC 19111 (lyophilized cultures of microorganisms, American Type Culture Collection, Kwik-stickTM set, MicroBioLogics) was used as a reference strain. The stock cultures were stored at –80 ºC in Tryptone Soya Broth supplemented with glycerol (15%). These strains were revitalized from frozen stocks by cultivation on Nutrient Agar (NA) plates and incubated 2 days at 37 ºC before performing the biofilm formation assays.
Biofilm formation assay
Biofilm formation assays were performed as previously described by Mowat et al. (2007) with a few modifications. Prior to testing, strains were grown on NA plates at 37 ºC for 48h. After the incubation, a loopful of actively growing cells was suspended in the appropriate medium TSYEB (HiMedia) or BHI (HiMedia) and adjusted to 0.5 McFarland standard turbidity to achieve a final cell concentration of 1 × 107 cells/ml. The assay was initiated by the addition of 200 µl cell suspensions into 96-well polystyrene microtiter plate, which were than incubated at four temperatures 7 ºC, 25 ºC, 37 ºC, 42 ºC for 5 days. In all experiments a positive (assay medium with bacterial strains) and a negative control (growth medium without bacterial strains) were included.
After incubation period, non-adherent cells were removed by washing three times with 250 µl sterile distilled water. After 10 min drying with hair drier, the bacterial cells in the wells were stained with 100 µl 0.5% crystal violet and left on bench for 20 min. The redundant crystal violet was removed by inverting the plates and the wells were washed three times with sterile distilled water and dried for another 10 min with hair drier. After adding 100 µl of 33% acetic acid into each well, the plates were shaken for 3 min to release the dye from the cells. The amount of adhered cells, i.e. the concentration of the released crystal violet was determined by measuring the optical density at 630 nm (OD630) using a microplate reader (ChemWel, Awareness Technology).
Statistical analysis
All quantitative data are presented as mean values with error represented by standard deviation (SD) from two independent experiments. The resulting data were analyzed using Anova: Two-Factor with replication in Microsoft Excel. A P-value of <0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
It is commonly accepted that cells in biofilms are more resistant to biocides, antibiotics, antibodies, and surfactants than are planktonic cells. Therefore, knowledge on biofilm capacity of foodborne pathogens is of major importance for the food industry, in order to define the most effective cleaning and disinfection strategies. Several L. monocytogenes isolates from meat were studied concerning their ability to produce biofilms at different growth conditions. The focus was on growth medium and temperatures (Moltz and Martin, 2005).
Evaluation of biofilm formation by L. monocytogenes in this study revealed that these bacteria possess a capacity for biofilm formation on polystyrene surfaces, in terms of the number of biofilm producing strains. However, it was noted that L. monocytogenes ATCC 19111 and Lm 1 were showed a much greater propensity for biofim formation in comparison with other strains (Figure 1 and Figure 2). This intra-species variation by L. monocytogenes in its adherence to plastic surfaces has been reported previously (Chavant et al., 2002; Djordjević et al., 2002; Stepanović et al., 2004). Such findings undoubtedly reflect inherent physiological differences between strains, and could have significance with respect to pathogenic potential.
 Figure 1. Biofilm formation by nine L. monocytogenes strains grown in TSYEB medium. Strains were incubated at 7 ºC, 25 ºC, 37 ºC, 42 ºC for 5 days. Each bar represents the mean value of the optical density (OD) ± standard deviation (SD). The experiments were performed with eight independent replicates
Figure 1. Biofilm formation by nine L. monocytogenes strains grown in TSYEB medium. Strains were incubated at 7 ºC, 25 ºC, 37 ºC, 42 ºC for 5 days. Each bar represents the mean value of the optical density (OD) ± standard deviation (SD). The experiments were performed with eight independent replicatesBacteria can grow over a wide range of temperatures and because adherence precedes growth, it is not suprising to find that biofilm formation also occur at wide temperature range. The influence of temperature on the ability of L. monocytogenes isolates to form biofilms has been previously reported (Norwood and Gilmour, 2001; Chavant et al., 2002; Di Bonaventura et al., 2008; Smoot and Pierson, 1998). Chavant et al. (2002) showed that L. monocytogenes LO28 colonized a polytetrafluoroethylene (PTFE) surface at 37 ºC, but not at 8 ºC. Di Bonaventura et al. (2008) also demonstrated that biofilm production on polystyrene surfaces by 44 different isolates of L. monocytogenes was significantly higher at 37 ºC than at 4 ºC. Norwood and Gilmour (2001), however, reported two L. monocytogenes isolates that adhered equally at 4 ºC and 30 ºC. In agreement with previous studies, we demonstrated that the biofilm formation of L. monocytogenes strains was significantly stimulated at 25 ºC, 37 ºC and 42 ºC in comparison to the lowest incubation temperature 7 ºC (P < 0.05). On the other hand, the results of the evaluation of biofilm formation on polystyrene surfaces by L. monocytogenes strains cultivated in two different media revealed that all tested strains produced biofilm in suitable medium. As shown in Figure 1 and Figure 2, the nutrient content of the medium significantly influenced the quantity of biofilm produced by tested bacteria (P < 0.05). The most effective medium in promoting biofilm production by the L. monocytogenes isolates from meat was BHI medium. On the contrary, statistical analysis showed that reference strain L. monocytogenes ATCC 19111 had a greater ability to form biofilm in TSYEB medium (P<0.05). BHI broth has been extensively used to study the biofilm formation capabilities of L. monocytogenes (Stepanović et al., 2004), and reported best biofilm formation of L. monocytogenes in BHI broth than other media studied (Kadam et al., 2013).
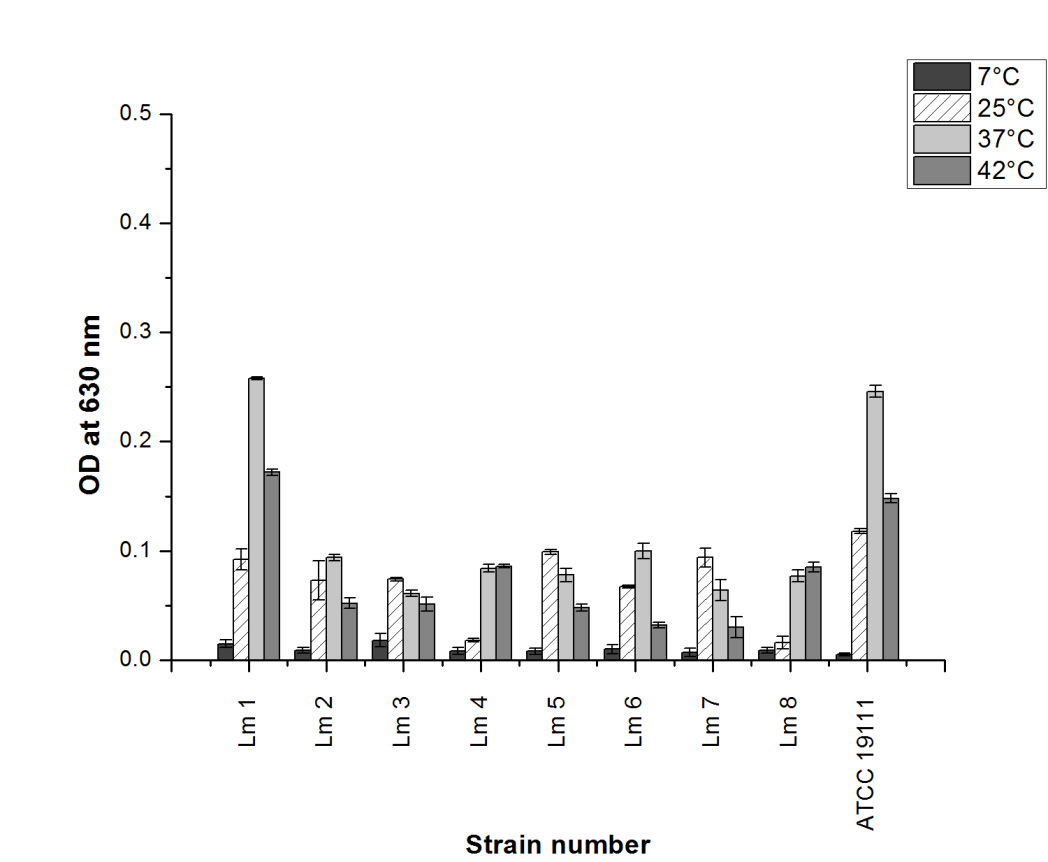 Figure 2. Biofilm formation by nine L. monocytogenes strains grown in BHI medium. Strains were incubated at 7 ºC, 25 ºC, 37 ºC, 42 ºC for 5 days. Each bar represents the mean value of the optical density (OD) ± standard deviation (SD). The experiments were performed with eight independent replicates
Figure 2. Biofilm formation by nine L. monocytogenes strains grown in BHI medium. Strains were incubated at 7 ºC, 25 ºC, 37 ºC, 42 ºC for 5 days. Each bar represents the mean value of the optical density (OD) ± standard deviation (SD). The experiments were performed with eight independent replicatesCONCLUSIONS
In conclusion, the incubation temperature was the most significant factor influencing the biofilm production levels. As we showed that L. monocytogenes strains were able to form biofilm at temperatures between 7 ºC and 37 ºC, a typical temperature used in the food industry during processing and storing, which suggests an increase in the likelihood of cross-contamination. For these reasons, our results could have a practical application to face the hygienic and sanitary issues raised by the adhesion in food industry by L. monocytogenes and indicate the importance of temperature as a factor. Furthermore, more information about biofilm formation characteristics of L. monocytogenes on food contact materials other than polystyrene with representative nutrient conditions will help to optimize strategies to control this biofilm-forming pathogen.
АCKNOWLEDGEMENTS
This paper is a result of the research within the project III46012 “Istraživanje savremenih biotehnoloških postupaka u proizvodnji hrane za životinje u cilju povećanja konkurentnosti, kvaliteta i bezbednosti hrane za životinje (Study of modern biotechnological methods in the production of animal feed in order to increase competitiveness, quality and safety of the feed)”, financed by the Ministry of Science and Technological Development, Republic of Serbia.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE