Mycobiota on common wheat (Triticum aestivum) and spelt (Triticum aestivum ssp. spelta) grains from the region of Vojvodina in 2015
ABSTRACT
INTRODUCTION
Wheat, as one of the most important grain crops in the world, can be easily grown in different climatic regions. Accordingly, wheat is susceptible to infections of many mycotoxin-producing mycobiota, such as Fusarium, Aspergillus, Penicillium and Alternaria (Alkadri et al., 2014). Fungal contamination of cereal crops causes yield losses, lower grain quality and results in the accumulation of mycotoxins in significant concentrations (Šarić et al., 2008). Mycotoxins may lead to health disorders due to a variety of their toxic effects on humans and animals. Wheat-based feed and food can be contaminated with mycotoxins in any stage of processing cycle: primary production, processing, transport and trading, i.e. “from field to fork” (Oliveira et al., 2014).
Spelt is an ancient wheat species currently receiving renewed interest due to its pro-health properties and suitability for organic production (Kurowski and Wysocka, 2009; Lacko-Bartošová et al., 2010). It is considered that spelt has a higher nutritional value than common wheat, but also higher resistance to unfavourable environmental factors including resistance to pathogens (Suchowilska et al., 2010; Solarska et al., 2012). Bodroža-Solarov et al. (2010) and Vučković et al. (2013) confirm that spelt hulls have protective effect and can reduce fungal and toxic contamination of spelt kernels, compared to common wheat kernels. Hard spelt hulls may pose an effective barrier for mycelial filaments of mycobiota.
The importance of species of the genus Aspergillus can be considered on the basis of their toxins that may have negative impact on food safety and quality (Gibbson, 2012). Currently, the most significant worldwide mycotoxins produced by Aspergillus spp. are aflatoxins, potentially carcinogenic and teratogenic metabolons for both humans and animals (Hedayati et al., 2007). Aspergillus species commonly occur in agricultural products in warm, arid, semi-arid and tropical regions where temperature and humidity conditions are suitable for the growth of mycobiota and production of toxins (Cotty and Jaime-Garcia, 2007). Data on the occurrence of Aspergillus species in Serbia indicate their generally low frequency and incidence in wheat grain (Lević et al., 2012a). Natural occurrences of aflatoxins are scarce in the agroecological conditions of Serbia, but there has recently been an intensified presence of these mycotoxins in maize (Kos et al., 2014). Lević et al. (2013) investigated the frequency and incidence of Aspergillus flavus in kernels of soybean, maize, barley, sunflower and wheat in the period 2008-2012. An exceptionally high emergence of Aspergillus species occured in 2012. Hot and dry weather with prolonged drought during spring and summer have great influence on a contamination of grains with aflatoxins. If the temperature becomes much higher with more frequent drought periods, it can lead to more frequent occurrence of Aspergillus species and their toxins in plant species in Serbia and other parts of the world.
The aim of this investigation was to determine species compositions and abundance of mycobiota colonizing grains of winter T. aestivum and T. aestivum ssp. spelta, with a special focus on Aspergillus flavus. The present study was also carried out in order to isolate and identify the potentially aflatoxigenic A. flavus in wheat.
MATERIALS AND METHODS
Fungal isolation and determination
The study was conducted on grain samples of T. aestivum and T. aestivum spp. spelt collected during the wheat harvest period (June-July, 2015) in the region of Vojvodina (Northern Province of Serbia).
Isolation and identification of fungi was performed according to Lević et al., (2012a). A hundred of common wheat kernels and fifty spelt kernels were taken randomly from each sample, being surface disinfected with 1% sodium hypochlorite for 5 minutes, and then rinsed three times with sterilized distilled water. The grains were dried on sterilized filter paper and placed on DG 18 - Dichloran 18% Glycerol agar (Himedia, India) media in five replicates for each sample. Each replicate contained 20 common wheat kernels and 10 spelt kernels per Petri dish. Plates were incubated at 25 °C for 7 days in darkness. Cultures obtained from growing colonies on DG 18 were transferred individually on PDA - Potato Dextrose Agar (Himedia, India) for futher purification and identification. In order to determine individual species of mycobiota reliably, fragments of the colonies developed on PDA were transferred to SNA (Syntetic Nutrition Agar) (Nirenberg and O’ Donnell, 1998), Aspergillus flavus and parasiticus agar - AFPA (Rodrigues et al., 2007) and Czapek’s agar (CZA). All cultivation, except on AFPA, were performed 7-14 days at 25 °C. On AFPA, selective medium for the identification of species from A. flavus group, cultivations were done at 30 °C for 3 days. Determinations of other fungal genera and species were done according to the following authors: Ellis (1971), Domsh et al. (1980), Travassos and Lioyd (1980), Nelson et al. (1983) and Samson and van Reenen-Hoekstra (1988). The frequency (F) and incidence (I) of mycobiota were calculated according to Lević et al. (2012a): F (%) = [Number of infected samples/Total number of samples] x 100; I (%) = [Number of infected kernels in the sample/Total number of kernels in the same sample] x 100.
Screening of toxin production
The Aspergillus isolates were cultivated on the standard yeast extract-sucrose agar (YESA - 2% yeast extract, 15% sucrose and 2% agar, pH 6.5, Samson and van Reenen-Hoekstra, 1988). The toxigenic potential of the isolates was determined after 14, 21 and 28 days of cultivation at 25 °C. The agar plugs were cut out of the colony centre with a sterile metal borer (diameter 6 mm), removed from the agar plate and placed with a sterile needle or tweezers in a sterilized Petri dish with a mycelial side up. The circular plugs were wetted with 10-20 μl of chloroform/methanol (2:1 v/v) and after few seconds the rapidly extracted mycelial side was gently applied against the TLC plate (Alugram SIL G/UV 254, Macherey-Nagel) with sterile tweezers. After drying the application spot, another one of the same colony was applied nearby, together with 30 μl of the working standard of the tested mycotoxin (internal standard). The thin-layer chromatography was performed in saturated tanks with toluene/ethyl acetate/formic acid developing solvent (90+10 v/v). After developing plates and air drying in a dark fume extractor, the plates were examined under long wave UV light (366 nm) (Bočarov-Stančić et al., 2009a).
RESULTS AND DISCUSION
Mycobiota on wheat grain
The presence of fungal genera in grain samples of T. aestivum and T. aestivum ssp. spelta was investigated during the wheat harvest period in 2015.
|
Fungal genera |
Frequency (%) |
Incidence (%) Mean value |
|
Alternaria spp. |
100.0 |
41.7 |
|
Arthrinium phaeospermum (Corda) M.B. Ellis |
20.0 |
1.0 |
|
Aspergillus flavus Link ex Fr. |
40.0 |
2.0 |
|
A. fumigatus Fres. |
20.0 |
1.0 |
|
A. nidulans G Winter |
20.0 |
1.0 |
|
A. niger van Tieghem |
20.0 |
1.0 |
|
Cladosporium spp. |
80.0 |
7.7 |
|
Dematiaceous Hyphomycetes (non-sporulating) |
100.0 |
16.2 |
|
Fusarium graminearum Schwabe |
100.0 |
13.2 |
|
F. poae (Peck) Wolenw. |
20.0 |
1.0 |
|
F. sporotrichioides Sherb. |
20.0 |
1.0 |
|
Gilmaniella humicola G.L. Barron |
20.0 |
2.0 |
|
Nigrospora oryzae (Berk and Br.) Petch. |
40.0 |
1.5 |
|
Penicillium spp. |
20.0 |
3.0 |
|
Sporotrix sp. |
20.0 |
1.0 |
|
Stemphylium spp. |
20.0 |
17.0 |
|
Trichocladium pyriforme Dixon |
20.0 |
2.0 |
|
Ulocladium spp. |
40.0 |
6.5 |
Mycological investigations showed the presence of 12 fungal genera on common wheat and 5 genera on spelt grains. The mycobiota isolated from the common wheat grains are shown in Table 1. Arthrinium sp., Aspergillus spp., Cladosporium spp., Gilmaniella sp., Nigrospora sp., Sporotrix spp., Trichocladium sp. were genera that were isolated from the common wheat grains, but not from the spelt (Table 2).
Table 2. presents infection frequencies and incidence of mycobiota in the spelt grain samples. More genera of mycobiota were isolated from the common wheat than from the spelt grains. Due to a higher stalk and hard adherent hulls, spelt is one of the cereals less infected by mycobiota (Solarska et al., 2012). Hulls might be considered as physical barriers that protect kernels from pathogens (Bavec et al., 2006). This might explain why more genera of mycobiota were isolated from the common wheat grains.
|
Fungal genera |
Frequency (%) |
Incidence (%) Mean value |
|
Alternaria spp. |
100.0 |
32.4 |
|
Dematiaceous Hyphomycetes (non-sporulating) |
100.0 |
22.0 |
|
Fusarium graminearum Schwabe |
100.0 |
10.4 |
|
Penicillium spp. |
40.0 |
2.0 |
|
Stemphylium spp. |
20.0 |
7.0 |
|
Ulocladium spp. |
20.0 |
8.0 |
Genus Alternaria showed the highest frequency (100.0%) on the common wheat grains (mainly A. alternata (Fr.) Keissl.) as well as on the spelt grains (mainly A. tenuissima (Nees) Wiltshire) with the incidence of 41.7% and 32.4%, respectively. Both mycobiota are typical saprophytic cosmopolitan opportunistic plant pathogens that cause grain discoloration, including developing of black spots (Šarić et al., 2008; Kurovski and Wysoska, 2009). According to Balaž et al., (2003), Stanković et al., (2007), Krnjaja et al., (2011), the presence of genus Alternaria (Figure 1) varies depending on agroecological conditions in Serbia for the relevant year of examination. The weather conditions in 2015 were characterized by moderate conditions and near-average precipitation across most of Serbia. Mean air temperature was in the categories of normal (Republic Hydrometeorological Service of Serbia, 2015). According to the results of other researchers of Alternaria mycobiota in Serbia, species that are usually detected on common wheat were A. alternata and A. logipes (Ivanović et al., 2001), while prevalent species on spelt wheat were A. alternata and A. tenuissima (Vučković et al., 2012), although A. infectoria was also observed (Đisalov et al., 2015). Under favourable conditions these species can produce different mycotoxins (alternariol, tenuazonic acid etc.) harmful to human and animal health (Vučković et al., 2013; Janjić-Hajnal et al., 2015).
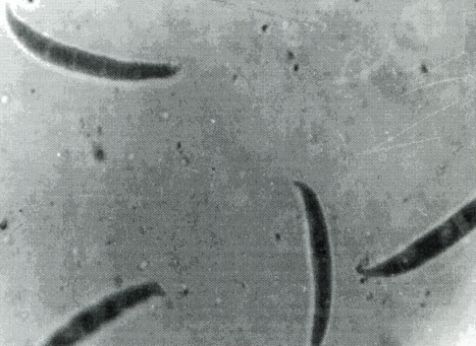
According to some authors, the presence of Fusarium spp. (mainly F. oxysporum) have considerable importance because it is the most often (found in about 45% samples) determined fungal genus on wheat in Serbia (Stojanović et al., 2005b). On the other hand Krnjaja et al. (2011) observe that other Fusarium species – F. graminearum (Figure 2a) and F. poae (Figure 2b) occured in winter wheat in a great abundance. In our investigation (Tables 1 and 2), the predominant fusaria on wheat grains (frequency 100%, incidence 13.2%) was also F. graminearum (96.0%), while other two identified species F. poae and F. sporotrichioides amounted to only 20% frequency and 1.0% incidence (Table 1). All samples of spelt grains were also colonized by F. graminearum, but the incidence of this mycobiota was lower than that on the wheat grains (Table 2). Although identified Fusarium species are weak cereal pathogens (with the exception of F. graminearum), all three of them can cause serious health disorders in humans and animals as producers of several mycotoxins (Bočarov-Stančić et al., 2007; Bočarov-Stančić et al., 2009b).
a) Fusarium graminearum b) Fusarium poae
Although the mycobiota of the genus Cladosporium had a high frequency (80.0%), their incidence was rather low (7.7%) on the common wheat (Table 1). Representatives of this genus, together with Nigrospora oryzae, are common mycobiota of wheat grain all over the world (Fahrunnisa et al., 2006; Lević et al., 2012b).
Unlike non-sporulating Dematiaceous Hyphomycetes (frequency 100.0%, incidence 16.2-22.0%), the sporulating ones (Gilmaniella humicola, Trichocladium pyriforme, Stemphylium spp. and Ulocladium spp.) were less frequently identified (20.0-40.0%) on both tested small grain cereals (Tables 1 and 2).
The potentially toxigenic species of Aspergillus and Penicillium genera had very low incidence (2.0-3.0%) and did not show too high frequency (Tables 1 and 2).
The incidence of Arthrinium phaeospermum and Sporotrix sp. was less prominent and amounted to only 1.0% (Table 1). A. phaeospermum is common mycobiota in both soft and durum wheat grains (Abdullah and Atroshi, 2014) as well as in winter spelt grains (Kurovski and Wysocka, 2009). Sporotrix sp. was previously found on wheat rachides in Serbia (Lević et al., 2012b) but not on wheat grains.
The results of microbiological analyses of wheat grain samples are in accordance with previously published results of other Serbian authors (Stanković et al., 2007; Krnjaja et al., 2011; Lević et al., 2012b).
Occurrance of Aspergillus flavus
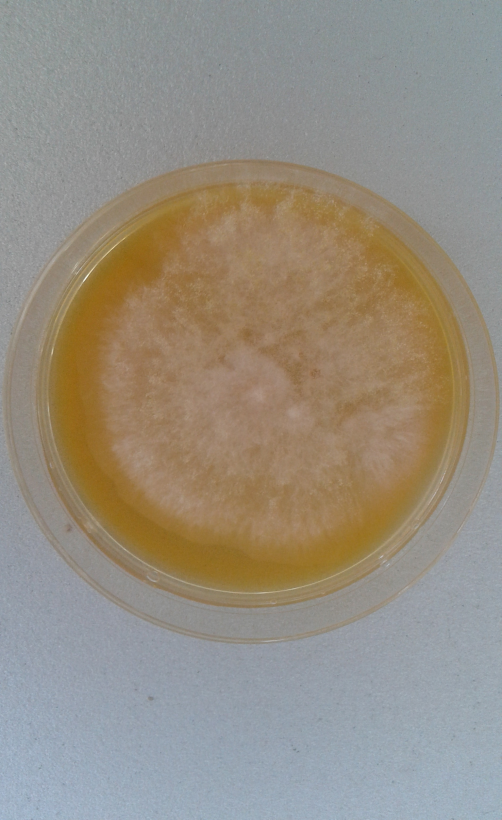
Aspergillus flavus (Figure 3) is mostly considered as a storage fungus, but under specific agroecological conditions it might cause high infection frequencies of grains in the field (Lević et al., 2013). In this study, a medium frequency (40.0%) and a low incidence (2.0%) of A. flavus on wheat samples was observed (Table 1). Similar occurrence of genus Aspergillus (1.4%) on wheat was noted in the investigation of Stojanović et al. (2005a).
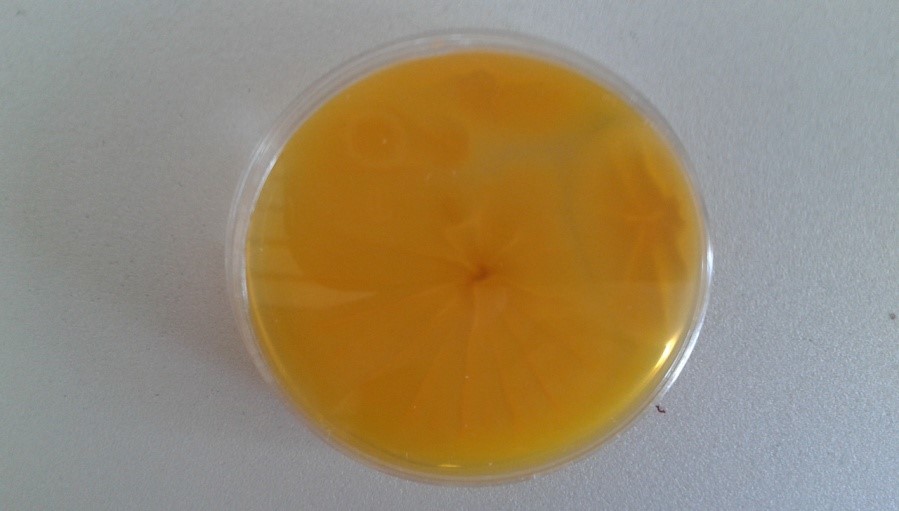
The type of isolation media for mycobiota has a special importance for the identification of A. flavus. AFPA (Aspergillus flavus and parasiticus agar) and Czapek’s agar (CZA) were used as selective media for the identification of strains from A. flavus group. These media are suitable for rapid detection and enumeration of A. flavus. Colonies of A. flavus in AFPA, incubated at 30 °C for 3 days are shown in Figure 4. The distinguishing between Aspergillus spp. is based on the development of characteristic orange colour on the reverse of the colonies cultivated on the AFPA plates (Rodrigues et al., 2007).
The toxigenic potential of Aspergillus flavus
The most important mycotoxins produced by mycobiota of the genus Aspergillus are aflatoxins, classified as primary carcinogenic compounds by the International Agency for Research on Cancer (IARC, 2002). Aflatoxin B1 (AFB1) is the most frequent and the most potent toxin.
Potential for the biosynthesis of AFB1 was investigated in 4 A. flavus isolates from the common wheat. Unlike the common wheat, A. flavus was not found on the samples of spelt wheat. The results gained with TLC showed that two isolates of A. flavus (12 and 201) had toxigenic potential for the AFB1 biosynthesis after 14 and 21 days of cultivation on YESA at 25 °C (Table 3). The AFB1 was not detected in any isolates after 28 days of cultivation.
|
Isolates designation |
The toxigenic potential of Aspergillus flavus |
||
|
14 days |
21 days |
28 days |
|
|
12 |
+ |
+ |
- |
|
73 |
- |
- |
- |
|
201 |
+ |
+ |
- |
|
202 |
- |
- |
- |
„+“- low intensity of biosynthesis; „-“- no biosynthesis
These results point out the necessity for monitoring the toxigenic potential and emergence of A. flavus in the field and also during storage and processing of small grain cereals. As a consequence of climate changes, stressful weather conditions (high temperatures and drought) may lead to production of aflatoxins, which can be associated with human and animal health risk.
CONCLUSION
The grain samples of common wheat were colonized by a higher number of fungal genera (12), then the spelt grain samples (5). Although the frequency of the determined mycobiota was similar on the common wheat grains and the spelt grains, there was a significant difference in their incidence. The present study shows the necessity for continuous mycological and mycotoxicological control of small grain, after harvesting, warehousing and processing, as one of the main steps in the evaluation of quality and health status of grains related to food safety. Changes in climatic conditions may lead to changes in mycobiota and mycotoxins commonly present in European samples of small grains.
АCKNOWLEDGEMENTS
This paper is a result of the research within the project III 46005 „New products based on cereals and pseudo-cereals from organic production”, funded by the Ministry of Education, Science and Technological Development, Republic of Serbia.

 JOURNAL TOOLS
JOURNAL TOOLS





 INSTITUTE
INSTITUTE