Rdar morphotype - a resting stage of some Enterobacteriaceae
2University of Novi Sad, Institute of Food Technology, 21000 Novi Sad,
Bulevar cara Lazara 1, Serbia
ABSTRACT
In this article, the characteristics of rdar and non-rdar morphotypes of diverse Salmonella serotypes isolated from animal feed, as well as Escherichia coli isolates originating from cow¢s milk with clinical mastitis, were presented. The research indicates substantial importance of the rdar phenotype for the persistence of Salmonella in the environment and its entering the food chain as well as the potential role of rdar phenotype of Escherichia coli in the pathogenesis of recurrent coli mastitis in dairy cows.
INTRODUCTION
Physiology of free floating bacteria was the subject of intensive studies during the twentieth century. However, lately a new view on the life of microorganisms is generally accepted. Under certain conditions, bacteria prefer a sessile way of life, as multicellular communities called the biofilm. Inside the biofilm, colonies of microorganisms are embedded in an extracellular substance which has the purpose to concentrate nutrients from the environment, to connect microcolonies and strengthen their interaction with the surface (living tissues or abiotic surfaces) having also multiple protective roles such as protection of microorganisms from desiccation, UV radiation, antibiotics, disinfectants and the effectors of the immune system. The composition of the extracellular matrix depends on the species of bacteria inside the biofilm, as well as the environmental conditions (Jonas et al., 2007). Biofilms are usually communities with different types of microorganisms, however when it comes to pathogens which are of particular interest to human and animal health, monospecies biofilms are mainly examined in experimental conditions. The formation of biofilm in Salmonella and Escherichia coli is part of their natural life cycles. Rdar morphotype, the subject of this paper, is the synonym for multicellular communities of bacteria whose main feature is their elevated biofilm production ability on abiotic surfaces (Römling, 2005). The occurrence of the rdar morphotype is essential for the survival of pathogenic bacteria outside of the host organisms, but is also significant for the pathogenesis of certain infections.
The name „rdar“ comes from the distinctive colony appearance which is formed by bacteria from the family Enterobacteriaceae on agar supplemented with the Congo red dye. When the bacteria isolates have the ability to form multicellular communities, their colonies are dark red color, with rough surface and undulate margins (red, dry and rough). In order for the isolates of E. coli and Salmonella spp. to form a distinctive rdar morphotype instead of the usual smooth colonies, it is essential to possess the ability to synthesize components of the extracellular matrix of the biofilm: curli fimbriae and cellulose. Another important structure was recently found in Salmonella Enteritidis strains - the surface protein Bap A (biofilm-associated protein) (Latasa et al., 2005). Besides the celulose, an important polysaccharide component is also the colanic acid, and in some serotypes of Salmonella unknown polysaccharides have been found (Vestby et al., 2009).
RDAR MORPHOTYPE AND COMPONENTS SIGNIFICANT FOR ITS DEVELOPMENT
The rdar morphotype was first described by von Lingelsheim in 1913 (Römling, 2005), but its importance has been acknowledged only in the past two decades. The twentieth century has been marked with research using conventionally grown bacterial cultures under laboratory conditions which show single swimming cell phenotypes. However, bacteria can manifest a different type of lifestyle: as sessile, static forms in multicellular communities (biofilm) and this has opened an interest for research of rdar phenotypes and their gene regulation. The formation of the biofilm was first investigated in long-term laboratory-passaged descendants of the E. coli strain K-12 (Römling, 2005). It was noticed that its cultivation in laboratory conditions resulted in loss of the ability for full biofilm formation, which was later described in other commonly used laboratory strains, such as Salmonella spp. (Davidson et al., 2008). One of the factors that affect the loss of the biofilm producing ability and, consequently, the expression of the rdar phenotype within the bacterial strains frequently passaged in laboratory, is the loss of the function of RNA polymerase, sigma S (rpoS), which codes for the sigma factor σ38. For the gene expression involved in the stress response and hereby the rdar morphotype formation the function of rpoS is of vital importance (Davidson et al., 2008). The gene regulation mechanisms of the rdar morphotype formation were studied first on the model of human pathogens, S. Typhimurium ATCC 14028 and E. coli TOB1 (Römling, 2005). Rdar morphotype was later discovered in other bacteria from the Enterobacteriaceae familly which include Citrobacter spp., Enterobacter spp. and Klebsiella spp. The expression of this specific bacteria phenotype leads to a resting stage, which is comparable to the formation of spores in Gram-positive bacteria (Davidson et al., 2008).
Curli fimbriae. The main protein component of the extracellular matrix produced by Salmonella spp. and Escherichia coli are the curli fimbriae (amiloid fibers). The curli fimbriae were first discovered in the late 1980s in Escherichia coli strains that caused bovine mastitis (Barnhart et al., 2007; Beloin et al., 2008). The name „curli“ fimbriae is designated from a unique protein curlin, from which these filaments are formed. Curli fimbriae (alternatively called Tafi or thin aggregative fimbriae in Salmonella) play the key role in the initial adhesion on the biotic and abiotic surfaces and the early intercellular aggregation (Beloin et al., 2008). Synthesis of curli fimbriae is a temperature dependent phenomenon in Enterobacteriaceae. In Salmonella, they are usually visible under 30 °C (Gerstel and Römling, 2003; Bokranz et al., 2005), but some strains such as S. Typhimurium, can express in vitro thin aggregative fimbriae at 37 °C (Olsen et al., 1998). Genes which encode the synthesis of curli fimbriae are highly conserved in wild strains of E. coli, but not all strains are able to produce them. Also, the expression of the curli fimbriae at 37 °C is a rarely visible phenomenon in clinical isolates of the E. coli. Therefore enteropathogenic, enterotoxigenic and uropathogenic E. coli (EPEC, ETEC and UPEC) express curli fimbriae only at ambient temperatures, while the majority of human sepsis and clinical isolates manifest this feature at 37 °C (Barnhart et al., 2007; Van Houdt et al., 2005). Curli fimbriae from virulent strains of E. coli, allow the process of adhesion on cell molecules (in the infected host) such as fibronectin and laminin, and their affinity for MHC-I molecules (major histocompatibility complex (MHC) class I) has also been confirmed (Olsen et al., 1998). Curli fimbriae in E.coli consists of polymers of a single 15-kDa protein encoded by the curlin subunit gene csgA (Olsen et al., 1998).
Cellulose. In Salmonella spp. and pathogenic E. coli strains the second component of the matrix is the polysaccharide cellulose, a β-1-4-D-glucose polymer. Cellulose is the most abundant biopolymer in nature. The cellulose is the structural component of the cell wall in plants, while in bacteria it is an extracellular product which acts as a mechanical and chemical protection (Vestby et al., 2009). The extracellular Salmonella matrix also consists of the O-antigenic capsule (O-Ag-capsule), and other capsular polysaccharides and lipopolysaccharides (Steenackers et al., 2012). Synthesis of fimbriae and cellulose is the result of a complex regulatory network, and depends on the expression of transcriptional regulator CsgD (also described thin aggregative fimbriae gene D (AgfD) (Gerstel and Römling, 2003; Jonas et al., 2007). The expression of the CsgD is under the influence of variable outside factors such as temperature, pH, osmolarity, the availability of nutrients and oxygen (Gerstel and Römling, 2003). CsgD directly influences the synthesis of the curli fimbriae by transcriptional activation of the csgBAC operon, while the synthesis of cellulose is indirectly activated through the regulator AgfD regulated gene (AdrA) (Jonas et al., 2007). The expression of the curli fimbriae and cellulose in Salmonella spp. is most intense at temperatures under 30 °C, in conditions of low osmolarity, limited availability of nutrients and aerobic conditions (Gerstel and Römling, 2003; Solomon et al., 2005; Steenackers et al., 2012).
Congo Red binding assay
The examination of the rdar morphotype is most commonly performed on the agar with the addition of Congo red (40 mg/L) and Comassie briliant blue (20 mg/L). Agar is prepared using Luria Bertani broth (LB) without salt (Bacto Yeast Extract (5g/L), Bacto Tryptone (10g/L). Congo Red and Comassie briliant blue are separately prepared, autoclaved and added to the agar base cooled to 55 °C. Congo Red has characteristic spectrophotometric properties. The absorbance spectrum of Congo Red differs upon binding cellulose or curli, allowing the detection of the two components when present alone or in combination. Therefore, strains producing cellulose appear pink, dry and rough (pdar) (Figure 1-A), while production of curli fimbriae results in brown, dry and rough (bdar) colonies (Figure 1-B). When no matrix components are expressed, the phenotype is smooth and white (saw) (Figure 3). The different Congo Red morphotypes constitute a valuable tool to study regulatory networks underlying the expression of the two extracellular matrix components cellulose and curli fimbriae. Comassie briliant blue is not a necessary component in Congo Red agar, but it provides a more precise color discrimination in the four specific morphotypes, mainly discriminating rdar and bdar morphology from pdar.
A less-pronounced phenotype on Congo red agar: ras (red and smooth; curli only), bas (brown and smooth; curli only) or pas (pink and smooth; cellulose only) has been found in E. coli strains (Bokranz et al., 2005).
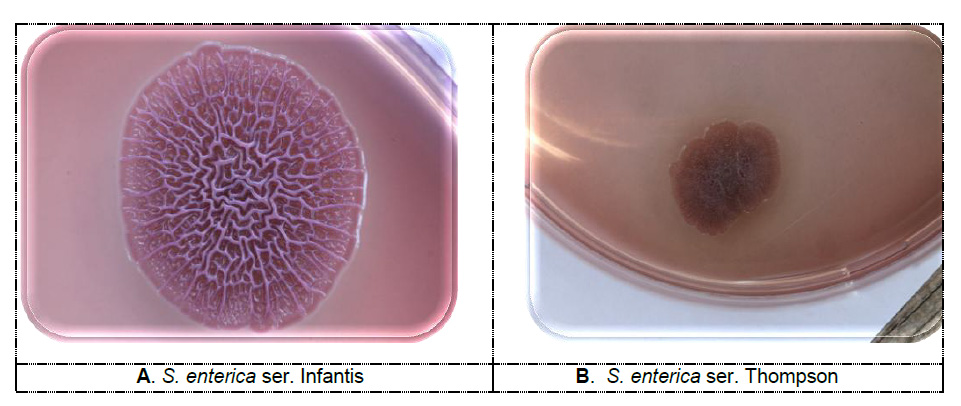 Figure 1. . Pdar (A) and bdar (B) morphotypes colonies of Salmonella enterica subsp. enterica serovars Infantis and Thompson on the Congo red agar, 5 days incubation at 20 °C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Figure 1. . Pdar (A) and bdar (B) morphotypes colonies of Salmonella enterica subsp. enterica serovars Infantis and Thompson on the Congo red agar, 5 days incubation at 20 °C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)(photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Multicellular behavior patterns in Escherichia coli isolates
In Escherichia coli strains isolated from cow¢s milk, which had clinical mastitis, we noticed different morphotypes on the Congo red agar, after incubation at 37 °C. The rdar phenotype was detected in certain isolates (Figure 2-A), as well as bdar, saw and mucoid (production capsules) morphotypes (Figure 2-B). The fact that some of the E. coli isolates, which cause bovine mastitis, produce both components of the extracellular matrix, especially the curli fimbriae at 37 °C, points to the possibility that these adhesive structures are involved in the pathogenesis of bovine mastitis (Karczmarczyk et al., 2008). Curli fimbriae are a virulence factor and they contribute to the adhesion and internalization of pathogens into epithelial cells. The infection of the mammary gland with these types of strains can result in persistency of the pathogens in tissue and recurrent clinical episodes of mastitis (Bradley et al., 2001; Passey et al., 2008). A specific E. coli pathogenic type, which has adapted to the mammary gland in ruminants has not been identified. Due to the fact that repeated episodes of coli mastitis do not always mean reinfections, but may be the result of pathogens persistence in the udder (in the form of biofilms), has recently resulted in drawing parallels between individual coli mastitis and pathogenesis of persistent infections caused by Staphylococcus aureus. Isolates of E. coli that produce the rdar morphotype may produce cellulose, but other polysaccharide components may be also responsible for the formation of the rdar morphotype, which is demonstrated in E. coli O157:H7 (Uhlich et al., 2006).
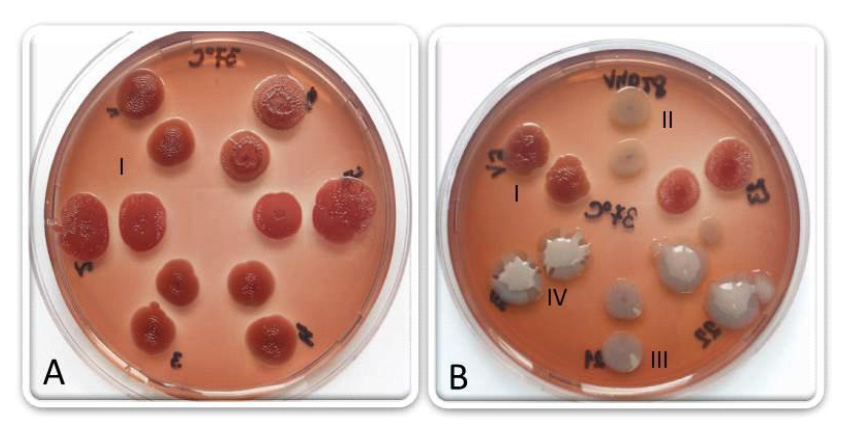 Figure 2..Morphotypes from different strains of Escherichia coli, isolated from cows milk with mastitis (72 h of incubation at 37 °C): rdar morphotypes (A); rdar, bdar, saw and mucoid morphotypes (B) (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Figure 2..Morphotypes from different strains of Escherichia coli, isolated from cows milk with mastitis (72 h of incubation at 37 °C): rdar morphotypes (A); rdar, bdar, saw and mucoid morphotypes (B) (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)(72 h of incubation at 37 °C): rdar morphotypes (A); rdar, bdar, saw and mucoid morphotypes (B)
(photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Multicellular behavour in Salmonella spp. isolates
Unlike the clinical isolates of E. coli, different serotypes of Salmonella isolated from animal feed, do not show the ability to form rdar morphotypes during incubation at 37 °C. At this temperature incubation, we only found the saw morphotype (Figure 3). This is in accordance with the fact that the expression of curli fimbriae in Salmonella can only be noticed at temperatures lower than 30 °C (Gerstel and Römling, 2003). The occurrence of saw morphotype at this temperature is most likely the consequence of the csgD transcriptional regulator deactivation.
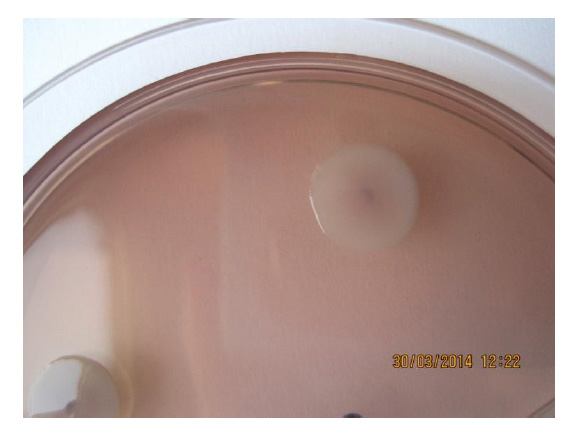 Figure 3. Saw morphotype of Salmonella enterica ser. Enteritidis, 5 days incubation at 37 °C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Figure 3. Saw morphotype of Salmonella enterica ser. Enteritidis, 5 days incubation at 37 °C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)It is observed that the invasive isolates of Salmonella such as S. Typhi (human adapted) and S. Choleraesuis (pig-adapted), that produce the saw morphotype, can avoid the defense of the host organism immune system (which may result in systemic infections) by not producing the curli and celulose (Steenackers et al., 2012). The cultivation of different serovars of Salmonella isolated from feed, on Congo red agar at 20 °C, resulted in forming of the characteristic rdar morphotypes (Figure 4, A-D).
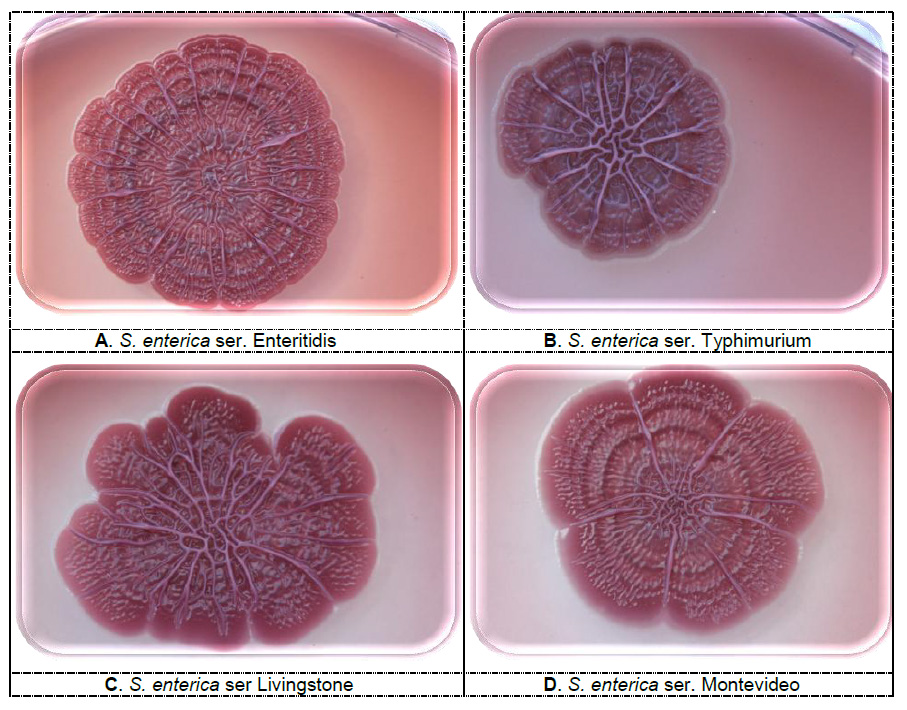 Figure 4. Rdar morphotypes of different serovars of Salmonella enterica, isolated from feed, 5 days incubation at 20°C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)
Figure 4. Rdar morphotypes of different serovars of Salmonella enterica, isolated from feed, 5 days incubation at 20°C (photo: Scientific Veterinary Institute “Novi Sad”, Serbia)Both Salmonella and Escherichia coli rdar and non-rdar phenotypes are present in nature, but it is believed that rdar is fundamentally important for the survival in the environment. The assumption is that the rdar morphotype is a critical factor for the transmission of Salmonella between susceptible hosts and it is a highly conserved property in the Salmonella enterica subgroup I isolates, that primarily infect warm-blooded hosts and are responsible for most human disease cases (White and Surette, 2006; Steenackers et al., 2012). The expression of the rdar phenotype is one of the ways that some clones of Salmonella persist in the feed factory environment for several years (Vestby et al., 2009). Rdar morphotypes of Salmonella spp. show greater resistance from long-term desiccation, nutrient depletion and the effects of disinfectants. Therefore, the production of curli, fimbriae and cellulose is essential for Salmonella survival outside the host, on plants (fresh fruit and vegetables) as well as in the food and feed manufacturing facilities (Solomon et al., 2005; White andSurette, 2006; Steenackers et al., 2012). The bdar strains are also able to survive and persist within adverse conditions, which was demonstrated in Salmonella serovar Agona strains isolated from feed producing facility (Vestby et al., 2009). Both morphotypes, rdar and bdar, are excellent biofilm producers, which indicates that cellulose is not the critical factor for the survival of Salmonella in unfavorable conditions, despite its protective role. The feed industry requires a dry production environment, which limits the growth of bacteria, but Salmonella has adapted to survive in such adverse conditions, due to their ability to form biofilm. Surface disinfection implemented by using chemicals at concentration effective against the population of bacteria in suspension, was not effective against the biofilm producing strains (rdar morphotype). For some strains of Escherichia coli, the rdar morphotype may ensure their survival and expansion in the environment, contamination of the milking system, and more recently its role in the pathogenesis of persistent coli mastitis in dairy cows has been considered (Bradley et al., 2001; Dogan et al., 2006).
CONCLUSION
Despite all the efforts that are being made to control the occurrence of salmonellosis in the human population, the number of infections has not decreased in the last decade (Brendanet al., 2013). Currently there are no effective strategy to combat the pathogenic bacteria which express rdar phenotype, and which are able to persist in the food and within a feed manufacturing environment. In addition, there is no effective therapy for infections caused by bacterial strains which produce biofilm. Therefore, rdar phenotype (biofilm) of pathogenic bacteria is a major research challenge in the field of food safety, human and animal health.
ACKNOWLEDGMENT
This work is supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (project number TR 31071).

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE