Presence of aflatoxins in cereals from Serbia
2University of Novi Sad, Faculty of Technology, 21000 Novi Sad, Bulevar cara Lazara 1, Serbia
3University of Novi Sad, Faculty of Agriculture, 21000 Novi Sad, Bulevar cara Lazara 1, Serbia
ABSTRACT
AFs content was determined by direct competitive Enzyme Linked Immunosorbent Assay (ELISA) method. Samples with AFs content higher than 1 µg/kg were analyzed again with confirmatory High Performance Liquid Chromatography with fluorescence detection (HPLC-FLD).
Analyses showed that none of the analyzed wheat (30), barley (20), oats (20) and rye (20) samples was contaminated with AFs. On the other hand, among 40 analyzed maize samples 24 of them (60%) were contaminated in the following way: 6 (25%) samples had AFs concentration between 1 and 10 µg/kg, 14 (58%) samples between 10 and 50 µg/kg and 4 (17%) between 50 and 70.3 µg/kg. The most predominant aflatoxin was AFB1 which was detected in all contaminated maize samples. AFG1, AFB2 and AFG2 were found in 12, 5 and 1 sample, respectively.
This study represents the first investigation of the occurrence of AFs in five different cereals from Serbia.
Introduction
Cereals are very susceptible substrate for the moulds growth. Depending on environmental conditions in the field and during storage, moulds growth may result in mycotoxin contamination of the crops. It has been reported that approximately 25% of total cereals world production is contaminated by at least one mycotoxin (Devegowda et al., 1998). Mycotoxins, especially AFs, are stable compounds and their degradation is limited at high temperatures. Therefore, mycotoxins can enter and contaminate the human food chain through cereal derived food products (Kabak, 2009).AFs are mainly produced by three Aspergillus species including A. flavus, A. parasiticus and A. nomius (Greppy, 2002). Contamination of plant materials by aflatoxigenic moulds may occur during pre harvesting, processing, transportation and storage (Ellis et al., 1991). Stack and Carlson (2003) reported environmental conditions, prolonged drought, high temperatures, substrate composition, storage time and storage conditions as the main influence factors for fungal growth and AFs synthesis. Furthermore, Aspergillus species and AFs are most commonly found in plant material such as cereals, grains and groundnuts from tropical and subtropical regions where temperature and humidity are optimum for the growth of moulds and production of the toxins (Rustom, 1997).
AFs are one of the main groups of mycotoxins due to their toxicity and prevalence. Those compounds are highly toxic, mutagenic, teratogenic and carcinogenic compounds and International Agency for Research on Cancer classified AFs as primary carcinogenic compounds (IARC, 2012).
Since AFs could contaminate a wide variety of agricultural product, which presents an important part of food chain, they can cause a great number of toxic effects in human and animal organisms. Furthermore, the presence of AFs could also be a cause of great economical losses (EFSA, 2013).
Serbia is an agricultural country with estimated total planted area at about 3.5 million HA for all crops. Furthermore, in the recent years, Republic of Serbia represents one of the largest maize and wheat producers and exporters in Europe (Maslac, 2011; Maslac, 2012). Regarding that, control of AFs in cereals is of great importance. Many countries, including Serbia, have obligatory control systems and maximum residue levels (MRLs) of AFs in cereals. The regulation of Serbia (Serbian Regulation, 2011) on the control of mycotoxins was harmonized with the regulation of European Union (European Commission, 2006b) and adopted in 2011. The both regulations define obligatory control of AFB1 and sum of AFs (AFB1, AFB2, AFG1 and AFG2). MRLs for AFB1 and AFs in cereals and cereals products are 2 and 4 µg/kg while these values for maize are slightly greater 5 and 10 µg/kg, respectively.
Due to the significant health risks associated with the presence of AFs in food chain, it is important to establish a data collection on the occurrence of these toxins in main cereals from Serbia. However, the presence of AFs in cereals from Serbia is very rarely investigated. The purpose of this work was to screen cereals for the AFs content since they represent an important part of human diet.
MATERIAL AND METHODS
Samples
Wheat (30), barley (20), oats (20), maize (40) and rye (20) samples were collected immediately after the harvest 2012 from the main cereals growing areas in Serbia. Sampling was performed according to EU requirements (European Commission, 2006a) in order to overcome uneven mycotoxins distribution among the crops and kernels. Certain numbers of incremental samples were combined in order to obtain aggregate samples of approximately 2-8 kg. Aggregate samples were homogenized and quartered to obtain a 500 g of laboratory samples which were afterwards ground to a 1 mm particle size using a laboratory mill (KnifetecTM 1095 mill, Foss, Hoganas, Sweden).Reagents and chemicals
Determination of AFs by ELISA was done using AFs Quantitative HS and AFs Quantitative Test kits (Neogen Veratox®, Lansing, USA). Chemicals used for ELISA analysis were methanol of analytical purity (Merck, Darmstad, Germany) and distilled water (Millipore, BedFord, MA, USA). For HPLC analysis acetonitrile, methanol, n-Hexane and trifluoroacetic acid (TFA) were purchased from Merck (Darmstadt, Germany). Used water was ultrapure (Milli-Q from Millipore, USA).Aflatoxins standard mix with certificated concentration of 1 µg/ml for AFB1 and AFG1, and 0.3 µg/ml for AFB2 and AFG2 was purchased from Sigma Aldrich (Prague, Czech Republic).
Determination of AFs by ELISA
Sample preparationSubsamples of 5 g were extracted with 25 mL mixture of methanol/water (70:30, v/v) and shaken vigorously for three minutes using laboratory Griffin flask shaker (Griffin and George, Wembley, England). Extracts were filtered through a Whatman No. 1 filter paper (Whatman International Ltd., Maidstone, UK). The obtained filtrates were collected, vortexed (Vortex mixer, Velp Scientifica) and used for further analysis.
ELISA procedure
AFs in the samples and standards are allowed to compete with enzyme-labeled AFs (conjugates) for the antibody binding sites. After washing, substrate is added, reacting with the bound conjugate to produce a blue colour. The intensity of the colour is inversely proportional to the concentration of AFs in the sample or in the standard. Intensity of the colour was measured at 650 nm in a microwell reader (Thermolabsystem, Thermo, Finland).
All cereal samples were analyzed with Quantitative AFs HS Test kit. Range of quantitation for this test kit is between 1 and 8 µg/kg. Samples with AFs concentration higher than 8 µg/kg were additionally analyzed with AFs Quantitative Test kit (range of quantitation 5-50 µg/kg). Samples with content of AFs more than 50 µg/kg were analyzed again after dilution.
Determination of AFs by HPLC
Sample preparationTwenty-five grams of maize sample were extracted with 100 ml acetonitrile:water (84:16, v/v) and shaken vigorously for thirty minutes using laboratory Griffin flask shaker (Griffin and George, Wembley, England). After extraction, an extract was filtered through filter paper (Whatman No. 4, Maidstone, UK) and 5 ml of filtered extract was cleaned up with Mycosep® 224 AflaZon multifunctional columns (Romer Labs. Inc., Union, MO, USA). The purified extract was then evaporated to dryness under air (Reacti Term, Thermo Fisher, Scientific Bellefonte, P. A. USA).
Since only AFB2 and AFG2 readily fluorescence, AFB1 and AFG1 must be derivatized prior to detection. Derivatization was achieved by adding 100 µl of TFA solution and 200 µl n-hexane to sample extracts or AFs working standards, vortexing for 30 s, and keeping them in the dark at 40 °C for 10 min. After evaporation, 400 µl of acetonitrile:water (1:9, v/v) mixture was added to the vials and vortexed again for 30 s.
HPLC conditions
The HPLC instrument was an Agilent 1200 system equipped with a fluorescence detector (FLD), a binary pump, a vacuum degasser, an autosampler and Agilent column (Eclipse XDB-C18, 1.8 µm, 4.6 x 50 mm). The AFs analysis was performed with a mobile phase consist of water (A) and a mixture of acetonitrile and methanol (50:50, v/v/v) (B) with a flow rate of 0.2 ml/min. The linear gradient program was applied from the beginning until 9 min with an increase of B from 35% to 45%. Further increase of B up to 70% was achieved in following 15 min, after which a B content was switched back to 35% in another 3 min. Post time was 2 min.
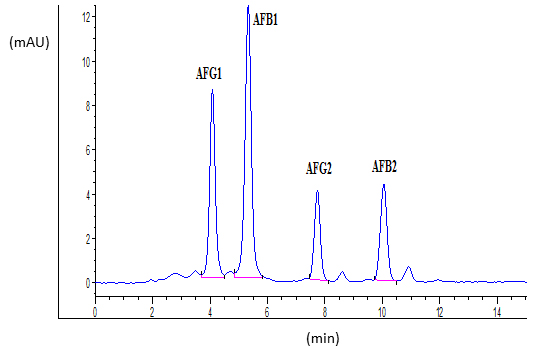
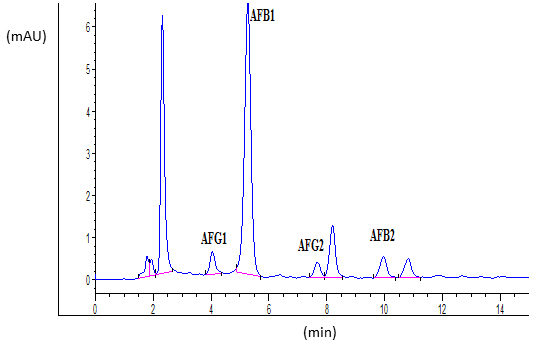
Ten microliters of standards or samples were injected into the HPLC column. The fluorescence detector was set to an excitation and emission wavelengths of 365 nm and 440 nm, respectively. Obtained retention times were 4.0, 5.2, 7.7 and 9.9 min for AFG1, AFB1, AFG2 and AFB2, respectively. Typical chromatograms of AFs standard and naturally contaminated maize sample were shown in Figures 1 and 2, respectively.
Quality control
The analytical quality of the ELISA and HPLC methods was assured by the use of certified reference material (CRM). Naturally contaminated maize sample with certified AFs content of 7.3±0.9 µg/kg was used as CRM for validation. CRM was supplied by Trilogy Analytical Laboratory (Trilogy® Reference Material, A-C-274, Washington, USA).
Results and Discussion
The validation parameters for ELISA and HPLC methods (Table 1) were calculated and expressed using European Official Decision procedure (European Commission, 2002b) and their values were in accordance with recommendations given in Regulation of European Union (European Commission, 2006a). Recovery experiments were performed using CRM samples. Recovery for the HPLC method was calculated on concentration of AFB1 since it was the predominant aflatoxin in CRM.
|
|
Quantitative High Sensitivity Test |
Quantitative Test |
HPLC |
|
LOD (µg/kg) |
0.50 |
1.40 |
0.33 |
|
LOQ (µg/kg) |
1.00 |
5.00 |
1.00 |
|
Recovery (%) |
107.5 |
95.1 |
94.8 |
Results of 130 cereal samples analyzed for AFs are summarized in Table 2. It is evident that none of the examined samples of wheat, barley, oats and rye were contaminated with AFs. On the other hand, among 40 analyzed maize samples, 24 of them (60.0%) were contaminated with AFs. Results obtained using ELISA method showed that AFs concentration in contaminated maize samples were distributed in the following way: 4 (17%) samples had the concentration between 1 and 10 µg/kg, 13 (54%) samples between 10 and 50 µg/kg and 7 (29%) between 50 and 75 µg/kg.
Cereals |
Number of analyzed samples |
Number/Percentage of samples contaminated with AFs |
|
Wheat |
30 |
- |
|
Barley |
20 |
- |
|
Oats |
20 |
- |
|
Maize |
40 |
24/60 |
|
Rye |
20 |
- |
Range of concentration (µg/kg) |
ELISA |
HPLC |
||||
|
CF |
CI |
CM |
CF |
CI |
CM |
|
|
1-10 |
17 |
1.15-5.83 |
3.66±2.01 |
25 |
1.05-8.92 |
5.34±2.57 |
|
10-50 µg/kg |
54 |
10.5-44.4 |
26.5±17.5 |
58 |
10.7-49.3 |
28.2±17.1 |
|
50-75 µg/kg |
29 |
50.0-75.0 |
64.0±11.9 |
17 |
55.6-70.3 |
63.6±6.31 |
Contamination frequency (CF, %), interval (CI, µg/kg), mean level and standard deviation (CM±SD, µg/kg)
ELISA method is defined as screening method which major advantages are minimal sample clean-up and preparation, simple procedure and low prices. However, the major disadvantage of ELISA method is possible cross-reactivity to similar compounds. Therefore, confirmation by HPLC based procedure is required (Anklam et al., 2002). HPLC analysis of contaminated maize samples confirmed that all 24 maize samples contained AFs. Results of AFs analysis using HPLC as well as the results obtained with ELISA method are shown in Table 3.
There is a slight difference between the results obtained with ELISA and HPLC methods. Differences in contamination frequency of analysed samples can be observed among the methods. Furthermore, HPLC analysis showed that AFB1 was present in all contaminated maize samples, AFG1 was present in 50%, followed by AFB2 in 21% and AFG2 in 4% of examined samples. This distribution of AFs in contaminated maize samples is in agreement with the results of Weidenborner (2011). Furthermore, AFB1 was reported as the most common aflatoxin (60-80% of the total aflatoxin content), with the remark that other AFs do not occur in the absence of AFB1. Also, in most cases, AFG1 could be found in a higher concentration than AFB2 and AFG2.
From the results obtained with confirmatory HPLC method it could be concluded that AFB1 and AFs concentrations were greater than MRLs of 5 and 10 µg/kg in 52.5% and 42.5% of examined maize samples, respectively. Regardless, some of the contaminated samples could be used as feed material if AFB1 concentration does not exceed 20 µg/kg according to European Union Regulation (European Commission, 2002a) or 30 µg/kg for AFs concentration according to Serbian Regulation (2014).
Weather condition changes during spring and summer 2012, described in our previous study (Kos et al, 2013), had a great influence on the occurrence of AFs in maize samples. Prolonged drought followed with hot and dry conditions, recorded in 2012, was favorable for growth of Aspergillus species and AFs synthesis. However, those weather conditions did not influence an occurrence of AFs in other examined cereal species. The possible reasons for the absence of AFs in wheat, barley, oats and rye samples can be found in different periods of growing season in comparison to maize as well as substrate composition. Period of maize planting, growing and harvesting (April-September, 2012) especially from June to September were characterized with extremely high temperatures and the lack of rainfall. Growing season for barley, wheat and rye usually last from October to May (USDA, 2010) and weather conditions during that period are mainly not favorable for Aspergillus species growth and AFs synthesis (Republic Hydrometeorological Service of Serbia, 2012). Although oats growing season is in the similar period as for maize, oats samples were not contaminated with AFs. Furthermore, carbon-rich substrates such as maize are favorable for growth of Aspergillus species where they can commonly be found as starchy food contaminants (Santin, 2005).
Results of our previous studies for the period 2008 - 2010 confirmed that AFs rarely occurred in cereals and cereals products from Serbia (Matić et al., 2008; Matić et al., 2009; Matić et al., 2010). In the recent years, the greatest numbers of published studies regarding AFs occurrence in cereals are from Mediterranean and Middle East, where the environmental conditions may favor the occurrence of Aspergillus species and AFs (Garrido et al., 2012; Karami-Osboo et al., 2012; Lutfullah and Hussain, 2012).
A report made by European Food Safety Authority (EFSA; 2013) in the terms of the occurrence of AFs in cereals and cereal-derived food products from Europe indicate low frequency of these mycotoxins. This study included 2183 samples from period 2007-2012 and showed that 70 (61 samples of rice) samples had AFs concentration greater than 1 µg/kg. Only in 6 (0.28%) samples detected levels of AFs were above the MRL. The most data in this report is collected from Germany, Austria, Slovenia and Ireland, however, it should be pointed that EFSA results did not include data from Serbia as well as from neighboring countries which had a similar problem with AFs contamination in maize harvested during 2012 year.
Beside presence of AFs in maize, weather condition changes during 2012 had a great influence on reduction in some cereals yield and production (Table 4). The greatest reduction in the yield was observed for maize, followed by oats and barley. Since the great amount of produced barley and maize in Serbia is intended for export, this decrease in yield influenced great economical losses.
Cereal |
Area harvested |
Yield |
Production |
Growth rate |
|
Wheat |
480 000 |
4 |
1900 |
-5.0 |
|
Barley |
78 000 |
3 |
269 |
-12.1 |
|
Oats |
50 000 |
2 |
85 |
-29.2 |
|
Maize |
1 300 000 |
3 |
3500 |
-45.3 |
|
Rye |
7000 |
2 |
12 |
0.0 |
Results are taken from: www.indexmundi.com/agriculture
Conclusions
Since examined five cereals species represent essential part of daily human diet, investigation of AFs presence is of a great importance. Obtained results showed that 60% of maize samples were contaminated with AFs in the concentration interval ranging from 1.05 to 70.3 µg/kg. Based on the results, continuous monitoring of AFs in maize is required. Although other investigated cereals were not contaminated with AFs, future control is necessary to avoid possible human exposure and other risks related to presence of AFs.
АCKNOWLEDGEMENTS
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Project No. TR 31029.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE