Selection of conditions for angiotensin–converting enzyme inhibition assay: influence of sample preparation and buffer
ABSTRACT
Introduction
Hypertension is one of the major chronic diseases. It is estimated that hypertension affects up to one third of the population in developed countries (Kearney et al., 2005). Untreated hypertension can lead to various ailments, including coronary heart disease, peripheral heart disease, stroke, and kidney dysfunction (Chen et al., 2009; Hernández-Ledesma et al., 2011).
One of the main regulators of blood pressure is angiotensin converting enzyme (ACE). ACE is a dipeptidilpeptidase containing zinc in its structure (Hernández-Ledesma et al., 2003). It acts on two body systems: renin-angiotensin (RAS) and kinin-kallikrein. ACE hydrolyzes decapeptide angiotensin I to octapeptide angiotensin II in lung capillaries. Angiotensin II is a potent vasoconstrictor and also increases aldosteron secretion that causes reabsorption of water and salts in kidneys. On the other hand, ACE inactivates vasodilator bradykinin, part of the kinin-kallikrein system (Chen et al., 2009; Hernández-Ledesma et al., 2011). Inhibition of ACE leads to decrease in angiotensin II production and increase in bradykinin, thus lowering the blood pressure (Chen et al., 2009). Different synthetic ACE inhibitors, such as Captopril and Enalapril, are widely used for treatment of hypertension (Hernández-Ledesma et al., 2011).
There are also natural ACE inhibitors and most of natural compounds that act as ACE inhibitors are protein hydrolysates and peptides obtained from animal and plant sources, predominantly cereals and legumes (Belović et al., 2011). In the last decades, many crude and purified plant extracts, as well as chemically defined compounds isolated from plants, have been evaluated for their ACE inhibitory activity (Barbosa-Filho et al., 2006).
There are different in vitro methods for the determination of ACE inhibitory activity. The most common method was developed by Cushman and Cheung (1971). This assay is based on the hydrolysis of hippuryl-L-histidyl-L-leucine (HHL) by ACE. The amount of hippuric acid formed in reaction is determined by measuring the absorbance at 228 nm (absorption maximum of hippuric acid). The difference between absorbance in the absence and presence of inhibitor is proportional to the inhibitory activity of tested sample.
This method has been modified in several ways by different authors. Buffer composition is often modified in addition to variations in ACE and HHL concentrations. Hernández-Ledesma et al. (2003) examined influence of different concentrations (0.1 M and 0.2 M) of borate and phosphate buffer on reaction rate (absorbance/min) as a function of HHL concentration. The highest reaction rate was obtained using 0.2 M phosphate buffer and it was selected for further studies. However, most authors used borate buffer in their investigation (Je et al., 2005; McCue et al., 2005). In papers published by Actis-Goretta et al. (2006) and Centeno et al. (2006), HCl-Tris buffer was used in reaction mixture, and the resulting hippuric acid was quantified by HPLC with UV detection. Sigma quality control test procedure uses HEPES sodium salt buffer as a reaction medium.
Samples for ACE inhibitory assay have been prepared in different ways. Liquid samples were usually subjected only to pH adjustment (Hernández-Ledesma et al. 2003; McCue et al., 2005; Centeno et al., 2006). Dry plant extracts were dissolved in HEPES assay buffer, or buffers with 10% ethanol or acetone (Duncan et al., 1999; Somanadhan et al., 1999).
The aim of this research was to test different buffers as reaction media and dried tomato extracts dissolved in different solvents in order to find the optimal conditions for performing the ACE inhibitory assay.
MATERIAL AND METHODS
Sample preparation
Lyophilized and grinded samples of commercially obtained tomato (4 g) were extracted with n-hexane (8 x 20 mL) in ultrasonic bath for 2 minutes. The residue was extracted with ethanol (40 mL) for 24 hours on shaker at room temperature. The solvents from both extracts were removed by evaporation in vacuum at 37°C, using rotary evaporator. Dried extracts were resuspended in ethanol, borate buffer (50 mM sodium borate and 500 mM NaCl, pH adjusted to 8.3 using 1 M HCl) and HEPES buffer (50 mM HEPES sodium salt and 300mM NaCl, pH adjusted to 8.3 using 1 M HCl) to a concentration of 1 mg/mL. These solutions were used for ACE inhibitory assay.
Reagents
Angiotensin converting enzyme (ACE) from rabbit lung (EC 3.4.15.1) and hippuryl-histidyl-leucine were purchased from Sigma (St. Louis, MO, USA). Captopril solution was prepared using procedure described by Donáth-Nagy et al. (2011) with some modifications. One tablet containing 25 mg of Captopril (Galenika, Belgrade) was pulverized in mortar and extracted with 25 mL of distilled water in ultrasonic bath for 10 min. Water extract was filtered through the filter with the pore size of 0.45 μm. Solution of captopril (1 mg/mL) was used as the positive control for ACE inhibitory activity (Duncan et al., 1999). Ethanol was used as the control for extracts dissolved in ethanol.
ACE activity assay
The ACE activity was determined by the modified method of Cushman and Cheung (1971). 50 µL of ACE solution (100 mU/mL) was incubated with 50 µL of borate buffer or HEPES buffer at 37°C for 10 min. After the addition of 150 µL of substrate (8.3 mM Hip-His-Leu in borate buffer or in HEPES buffer prepared as indicated above), the reaction mix was incubated for 80 min at 37°C. The reaction was terminated by the addition of 250 µL of 1 M HCl. The resulting hippuric acid was extracted with 3 x 500 µL of ethyl acetate and centrifugated at 800 g for 15 min. 750 µL of the upper layer was transferred into test tube and evaporated under air flow at 37°C. The hippuric acid was dissolved in 1 mL of distilled water, and the absorbance was measured at 228 nm using UV/Vis spectrophotometer (Cintra 303, GBC Scientific Equipment, Australia). The reaction blank was prepared in the same way indicated above, with change in the order in which the reagents were added (HCl was added before the enzyme).
ACE inhibitory activity assay
The inhibition percentages of the tomato extracts, Captopril and ethanol were determined using the method described above, replacing the 50 µL of buffer with the same volume of the samples dissolved at concentration of 1mg/mL. The sample blank was prepared in the same way as the reaction blank, replacing the volume of buffer by the tested sample. In order to eliminate the interferences in the analysis, ACE inhibition was calculated according to equation (Hernández-Ledesma et al., 2003):
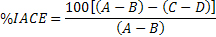
where A represents absorbance in the presence of ACE, B absorbance of the reaction blank, C absorbance in the presence of ACE and inhibitor, and D absorbance of the sample blank. All determinations were carried out in duplicate.
Data analysis
Microsoft Excel was used for calculating means and standard deviations. Analysis of variance (ANOVA) and Duncan's multiple range test were used to compare means at 5% significance level by using the statistical data analysis software system STATISTICA (StatSoft, Inc. (2011), version 10.0 (www.statsoft.com)).
Results and Discussion
The results of ACE inhibitory activity assay are presented in Table 1 and Table 2. There were no statistically significant differences between results obtained with borate and HEPES buffer. However, assays with borate buffer produced residues that were more easily dissolved in water after evaporation of ethyl acetate giving higher absorbance readings. Higher absorbance readings give greater difference between the test and the blank, which is important for samples with high interferences, such as samples used in this experiment. Therefore sodium borate buffer has been chosen as reaction medium for further investigations.
Tomato extracts dissolved in ethanol completely inhibited ACE, as well as ethanol. Results of ANOVA showed that there was no significant difference in inhibitory activity between the extracts and ethanol. It was concluded that extracts dissolved in ethanol could not be used for this assay because ethanol masked compounds with potential inhibitory activity.
Captopril showed similar values in all performed experiments. Complete inhibition of ACE by Captopril in concentration of 1 mg/mL is in concordance with results obtained by other authors. Duncan et al. (1999) established on the basis of literature the IC50 value of 17.7 nM for Captopril. Therefore, concentration of Captopril used in this assay is more than sufficient to completely inhibit ACE.
|
Solvent |
Tomato hexane extract |
Tomato ethanol extract |
Captopril |
Ethanol |
|
Sodium borate |
4.5±5.2 |
0.6±3.1 |
100.7±1.5 |
- |
|
Ethanol |
103.5±1.6 |
102.5±5.9 |
103.3±3.0 |
102.7±3.7 |
Results are given as mean ± standard deviation (n = 2). Concentrations of tomato extracts and Captopril were 1 mg/mL.
|
Solvent |
Tomato hexane extract |
Tomato ethanol extract |
Captopril |
Ethanol |
|
HEPES |
0.1±4.2 |
6.6±5.5 |
104.3±2.3 |
- |
|
Ethanol |
109.8±1.2 |
105.0±4.5 |
96.7±1.8 |
109.5±4.5 |
Results are given as mean ± standard deviation (n = 2). Concentrations of tomato extracts and Captopril were 1 mg/mL.
Conclusions
There were no statistically significant differences between results obtained by sodium borate buffer and HEPES buffer. After evaporation of ethyl acetate, enzymatic reaction products were more easily dissolved in water when borate buffer was used in the assay making absorbance readings more accurate and therefore it has been chosen for further experiments. Ethanol was not suitable for sample dissolution because it interfered with the samples and masked their inhibitory activity, so false positive results can be obtained.
АCKNOWLEDGEMENTS
This paper is a result of the research within the project III 46001 “Развој и примена нових и традиционалних технологија у производњи конкурентних прехрамбених производа са додатом вредношћу за европско и светско тржиште - СТВОРИМО БОГАТСТВО ИЗ БОГАТСТВА СРБИЈЕ (Development and utilization of novel and traditional technologies in production of competitive food products with added value for national and global market - CREATING WEALTH FROM THE WEALTH OF SERBIA)”, financed by the Ministry of Education, Science and Technological Development, Republic of Serbia.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE