FILAMENTOUS CYANOBACTERIA FROM VOJVODINA REGION AS SOURCE OF PHYCOBILIPROTEIN PIGMENTS AS POTENTIAL NATURAL COLORANTS
DOI:
UDK:
JOURNAL No:
Volume 39, Issue 1
PAGES
23-32
KEYWORDS
cyanobacteria, microalgae, natural colors, pigments, phycobiliproteins
Jelica B. Simeunović*1, Snežana B. Marković2, Dajana J. Kovač1, Aleksandra Č. Mišan3,
Anamarija I. Mandić3, Zorica B. Svirčev1
1 University of Novi Sad, Faculty of Natural Science, Trg Dositeja Obradovića 3, 21000 Novi Sad, Serbia
2Water supply and Sewerage Company of Novi Sad, Masarikova 17, 21000 Novi Sad, Serbia
3 University of Novi Sad, Institute for Food Technology in Novi Sad, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia
ABSTRACT
Phycobiliproteins are a group of colored proteins present in cyanobacteria (blue-green algae). They are extensively commercially used in foods, cosmetics, biotechnology, pharmacology and medicine. In order to determine production of phycobiliproteins in cyanobacterial strains, the quantity of these pigments in 10 filamentous cyanobacteria was investigated. The study was conducted with terrestrial cyanobacterial strains isolated from different soil types in Vojvodina region (Serbia) which belong to Nostoc, Anabaena and Spirulina genera. The obtained results showed that the qualitative and quantitative contents of phycobiliproteins depended not only on cyanobacterial strain, but also on the composition of growth media. In case of strains which belong to Nostoc and Anabaena genera higher pigment content was found in case of strains cultivated in nitrogen free medium compared to strains grown in medium with nitrogen. The highest content of phycocyanin was found in Anabaena strain C2 (22.62 µg/ml) grown in nitrogen free medium, while Nostoc strain S1 contained the highest PC amount of 18.37 µg/ml growing in presence of nitrogen. The Nostoc strain S1 had the highest content of allophycocyanin growing in both type of nutricious media (25.11 µg/ml and 13.88 µg/ml, respectively). The highest phycoerythrin concentration was characterized the strain Anabaena LC1B in nitrogen free medium (24.87 µg/ml) and in presence of nitrogen (20.85 µg/ml). The results of total phycobiliprotein content of tested cyanobacteria showed that the highest content in nitrogen free condition characterized Anabaena strains C2 (57.70 µg/ml) and LC1B (58.75 µg/ml) and Nostoc strain S1 (57.26 µg/ml). Compared to these Nostoc and Anabaena strains the contents of all three pigments as well as total pigment content were much lower in both tested Spirulina strains. Therefore, some studied strains of Anabaena and Nostoc genera represent excellent sources of one or more phycobiliproteins.
INTRODUCTION
Natural colorants for food are made from renewable sources. Most often, the colorants are extracted from plant material, but other sources such as insects, algae, cyanobacteria (blue-green algae) and fungi are used as well. Legislation restricts which colorants are allowed, what sources may be used for that particular colorant, what solvents may be used to extract it, and the purity of the pigment (Mortensen A., 2006; Eriksen, 2008). Natural colorants such as phycobiliproteins are gaining importance over synthetic ones, as they are nontoxic and non-carcinogenic. The natural colorants allowed in the EU and the USA are also allowed in most parts of the world (Mortensen A., 2006).
Cyanobacteria (blue-green algae) as specific group of microorganisms represent a potential source of commercially important chemicals and pharmaceutical products. Among them, phycobiliproteins are very interesting cell constituents with high commercial value. Because of their protein nature, unique color, fluorescence and other properties a wide range of promising applications of phycobiliproteins are possible (Zhao et al., 1995; Rossano et al., 2003, Sekar and Chandramohan, 2008). Due to the toxic and possible cancerogenetic effects of several synthetic dyes, there is an increasing preference to use natural colors such as phycobiliproteins (Seker and Chandramohan, 2008). These pigments can be used as natural colorants in food and drug industry and in cosmetic preparation replacing the synthetic dyes (Cohen, 1986: Soni et al., 2006). Phycocyanin isolated from cynobacterial species Spirulina platensis is widely used as a natural pigment in food, such as dairy products and jellies (Santiago-Santos et al., 2004), coated soft candies (Lone et al., 2005), fermented milk products, ice creams, deserts, milk shakes and sweet cake decoration (Sekar and Chandramohan, 2008). Use of phycobiliproteins for that purposes has to be supported with toxicity testing. Also, phycobiliproteins are applied as fluorescent markers in immunoassays, in biomedical research for cancer diagnostics and as therapeutics (Glazer, 1994; Soni et al., 2006). Moreover, recent studies have shown their immunomodulating and anticarcinogenic activities (Rossano et al., 2003) and their neuroprotective and hepatoprotective properties (Spolaore et al. 2006; Seker and Chandramohan, 2008). In global patent databases there are 297 patents of phycobiliproteins, and the majority of them are from USA, Japan and Europe (Sekar and Chandramohan, 2008). Patents from USA are mostly related to application of pigments as fluorescent dye while in Japan phycobiliprotein investigations and patents are focused on production, purification and application for therapeutic and diagnostic purposes (Sekar and Chandramohan, 2008). The greatest part of more than 3000 tons of Spirulina platensis dry weight is annually produced worldwide because of phycobiliproteins, which are used for health food products and animal feed additives (Spolaore et al., 2006).
Phycobiliproteins are the major photosynthetic accessory pigments in cyanobacteria which are brilliantly colored, water-soluble proteins, bearing covalently attached open chain tetrapyrroles (Patel et al., 2005). According to the color and absorption ability, phycobilins are divided in 4 main groups: phycoerythrin (PE) -red pigment, allophycocyanin (APC)- bluish green, phycocyanin (PC)-blue, and phycoerythro-cyanin (PEC) -orange pigment (Cohen-Bazire, Bryant, 1982). Phycocyanin is a phycobiliprotein that has been recently reported to exhibit a variety of pharmacological properties. In this regard, antioxidant, antiinflammatory, neuroprotective and hepatoprotective effects have been experimentally attributed to PC (Romay et al., 2003). Phycoerythrin can be used to mark antibodies and other biological elements in laboratory testing.
Phycobiliproteins are variably distributed in the representatives of the division of Cyanobacteria. Blue pigments, phycocyanin and allophycocyanin are present in all cyanobacteria, red pigment, phycoerythrin, is widely spread but it is not found in all cyanobacteria, while phycoerythrocyanin usually found in filamentous species (Hoffmann et al., 1990). The ratio of these pigments can be environmentally altered. Filamentous cyanobacteria are particularly attractive for the photoproduction of phycobiliproteins and other chemicals (Borowitzka, 1995). Since that it is of great significance to investigate phycobiliprotein production of especially filamentous cyanobacteria originating from different habitats.
In Serbia research in the field of cyanobacterial products such as phycobiliproteins and their commercial exploitation is very nascent and need adequate attention.
In this study ten strains of terrestrial filamentous cyanobacteria, isolated from different soil types in Vojvodina region, were screened for their potential as producers of phycobiliprotein pigments.
Analysis of the phycobiliprotein contents was performed on nine cyanobacterial strains originating from various terrestrial environments (solonetz, meadow black soil, chernozem, sand from the banks of the Danube river) in the Vojvodina region and one Spirulina strain SJ originating from Algal Culture Collection of Tokyo (Japan) (Table 1).
All tested cyanobacterial strains belong to 3 different genera, Nostoc, Anabaena and Spirulina. Determination of phycobiliproteins: phycocyanin (PC), allophycocyanin (APC) and phycoerythrin (PE) in cyanobacterial strains was carried out during their growth in the laboratory, under certain controlled conditions of temperature, light, mineral content. Pigment content was determined after 21th days (during the stationary phase of growth). Cyanobacterial strains were grown photoautotrophically in BG 11 medium with and without nitrogen (Rippka, et al., 1979), while Spirulina strains were cultivated in SOT medium (Soong, 1980). Cultures were maintained at 22-24 oC under illumination by cool white fluorescent light (50 µmol m-2s-1). In terms of duration of light and dark period is most commonly used mode 12 hours light and 12 hours of darkness.
Content of phycobiliproteins (PC, APC and PE) was determined using spectrophotometric method (Bennett, Bogorod 1973). Strains were grown in Erlen-Meyer vessels where the inocula (1 ml) were streaked in the medium (80ml). After 21 days of incubation, 10 ml of samples (cultures) were taken for the determination of phycobiliproteins and then each sample was centrifuged at 3000 rpm for 5 minutes. The collected cell mass was then washed with buffer 1 M Tris-Cl (pH 8.1). One volume of cell mass was then resuspended in five times of the volume of the same buffer. To extract pigments, it was necessary to make splitting the cell wall of cyanobacterial strains. The procedure of continuous freezing at -20 ° C and thawing at +4 ° C, and sonication of samples (10 minutes with cycles of 30 seconds), allowed the destruction of the cell wall of the strains. After that the separation of cell fragments by centrifugation at 12,000 rpm for 10 minutes was done.
All supernatants of tested cyanobacteria were then separated and concentration of pigments was determined spectrophotometrycally at wavelengths of ? = 562 nm for phycoerythrin (PE), ? = 615 nm for phycocyanin (PC) and ? = 652 nm for allo-phycocyanin (APC). Absorbance measurement was performed on the spectrophotometer "NICOLET Evolution 100" (Thermo Electron Corporation). Concentration of pigments was determined using the following formula:
PE [mg/ml] = (A562-2.41xPC-0.849xAPC)/9.62 PC [mg/ml] = (A615-0.474xA652)/5.34
APC [mg/ml] = (A652-0.208xA615)/5.09
Each sample was analyzed in duplicate and buffer was used as a blank.
Table 1. Tested cyanobacterial strains and their origin
|
Cyanobacterial strains |
Genus |
Origin |
|
|
C2 |
Anabaena |
chernozem |
|
|
C5 |
Anabaena |
chernozem |
|
|
2S9B |
Nostoc |
solonetz |
|
|
SS |
Spirulina |
Danube bank (Serbia) |
|
|
SJ |
Spirulina |
Japanese Culture Collection |
|
|
|
|
||
RESULTS AND DISSCUSION
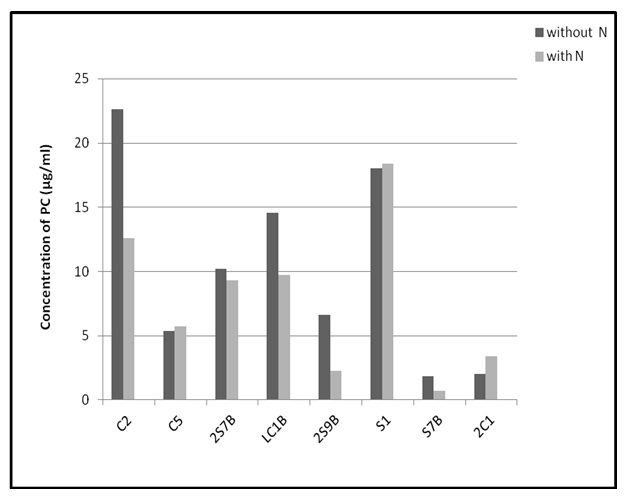
The results of PC concentrations in investigated cyanobacteria showed that higher contents of this pigment were found in most strains during the growth in nitrogen free medium, compared to presence of nitrogen (Fig 1). The lowest content was characterized Nostoc strain S7B (1.86 µg/ml), while the highest content of PC was found in Anabaena strain C2 (22.62 µg/ml). Also very high production of PC in nitrogen free condition was detected in Anabaena strain LC1B and Nostoc strain S1 with values of 14.57 and 18.03 µg/ml, respectively. Growing in presence of nitrogen, Nostoc strain S1 contained the highest PC amount of 18.37 µg/ml (Fig 1). Other tested strains had smaller contents of PC which were in the range of 0.70 (strain S7B) -12.61 µg/ml (strain C2).
The content of pigment allophycocyanin in almost all strains growing in nitrogen free medium was higher than during their growth in medium with nitrogen (Fig 2). Only two strains, 2S7B and 2C1 had higher content of this pigment when they grown in presence of nitrogen. The Nostoc strain S1 had the highest amount of APC (25.11 µg/ml). Also very high content was found in Anabaena strains such as LC1B (19.31µg/ml) and C2 (20.92 µg/ml). In the presence of nitrogen the highest concentration of APC was characterized Nostoc strain S1 (13.88 µg/ml). Very high content was also found in Anabaena strains C2, 2S7B and LC1B (13.18 µg/ml, 13.02 µg/ml, 10.63 µg/ml, respectively) (Fig 2). Other tested strains had content of APC less than 10 µg/ml.
The content of pigment allophycocyanin in almost all strains growing in nitrogen free medium was higher than during their growth in medium with nitrogen (Fig 2). Only two strains, 2S7B and 2C1 had higher content of this pigment when they grown in presence of nitrogen. The Nostoc strain S1 had the highest amount of APC (25.11 µg/ml). Also very high content was found in Anabaena strains such as LC1B (19.31µg/ml) and C2 (20.92 µg/ml). In the presence of nitrogen the highest concentration of APC was characterized Nostoc strain S1 (13.88 µg/ml). Very high content was also found in Anabaena strains C2, 2S7B and LC1B (13.18 µg/ml, 13.02 µg/ml, 10.63 µg/ml, respectively) (Fig 2). Other tested strains had content of APC less than 10 µg/ml.
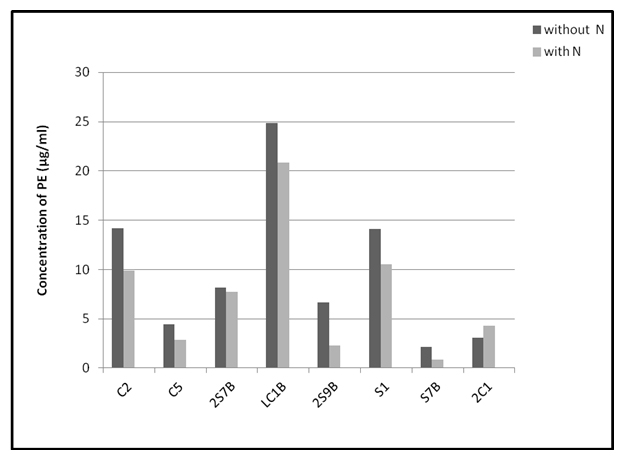
The content of red pigment phycoerythrin was higher in almost all tested strains during their growth in nitrogen free medium (Fig 3). Only strain 2C1 grown in presence of nitrogen had higher amount of this pigment compare to condition without nitrogen. Concentration of PE of strains grown in nitrogen free medium varied from 2.15 µg/ml (strain S7B) to 24.87 µg/ml (strain LC1B). Strains grown in condition with nitrogen showed lower production of phycoerithrin with concentrations in the range of 0.89 µg/ml (strain S7B) – 20.85 µg/ml (strain LC1B) (Fig 3).
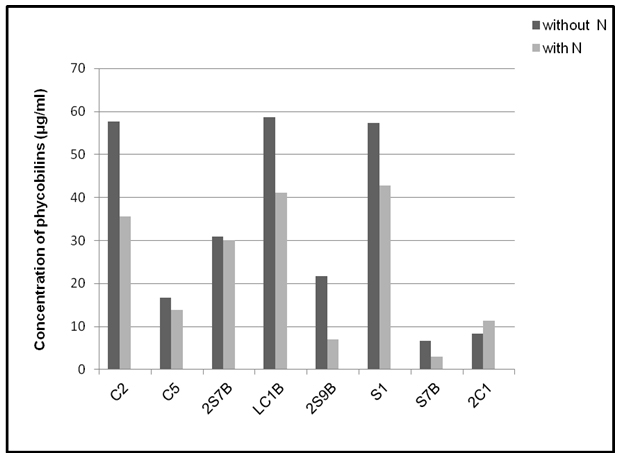
The results of total phycobiliprotein content of tested cyanobacteria showed that the highest contents in nitrogen free medium characterized Anabaena strains C2 (57.70 µg/ml), LC1B (58.75 µg/ml) and Nostoc strain S1 (57.26 µg/ml). The same strains had the highest content of phycobiliproteins during their growth in the medium with nitrogen compared to other tested strains (Fig 4). The lowest concentration of phycobiliproteins was found in Nostoc strain S7B, both during the growth in nitrogen free medium and in presence of this element.
The results showed that qualitative and quantitative content of total and individual phycobilin pigments was different, which clearly shows the existence of specific features in the pigment composition of every examined strain. The analysis of the distribution of phycobiliproteins in all strains, showed the presence of all three types of phycobilin pigments – PC, APC, PE in different proportions. Moreno et al. (1995) found that in some strains of Anabaena and Nostoc generathe prevalent type of phycobiliproteins was C-phyco-cyanin, followed by allophycocyanin, with levels of 17 and 11% d.wt, respectively, while C-phycoerythrin was the major pigment in several Nostocstrains, reaching 10% d.wt. In the present study most tested strains of Anabaena and Nostoc genera had the highest content of allophycocyanin (6 in nitrogen free medium and 4 in the presence in nitrogen), while the highest content of phycocyanin was found in one Anabaena strain in nitrogen free medium (C2) and in two strains in the presence of nitrogen (C5 and S1). The highest content of phycoerythrin was characterized in Anabaena strain LC1B in both condition and Nostoc strain 2C1 in the presence of nitrogen.
There is a lot of information about some average contents of phycocyanin, allophycocyanin and phycoerythrin which are found for some representatives of cyanobacteria. Metabolic examinations showed that an extract of cyanobacteria Fremyella diplosiphon contains from 35-40 mgml-1 of phycoerythrin, while an extract of Calothrix sp. biomass had 84 mgml-1 of phycocyanin (Santiago-Santos et al. 2004). Colyer et al. (2005) in the examinations of pigment and protein contents of cyanobacteria detected various concentrations of 3 phycobilins. The values varied in the range of 0.20-4.92 mgml-1 for allophycocyanin, 0.73-18.24 mgml-1 for phycocyanin and 0.77-19.30 mgml-1 for phycoerythrin. In the present study the results of phycobiliprotein content for the examined terrestrial cyanobacterial strains are very similar to the values which were found in these studies. But, some strains tested in our investigation, such as Anabaena strains C2 and LC1B and Nostoc strain S1 had higher contents of investigated pigments. Therefore, these filamentous strains represent promising sources of one or more phycobiliproteins. However, the detected concentrations of all examined pigments are significantly different from the values found by Soni et al. (2006) in the examination of phycobilin content of the species Oscillatoria quadripunctulata, which varied from 27.43 mgml-1 for phycocyanin, 15.80 mgml-1 for allophycocyanin and 0.45 mgml-1 for phycoerythrin.
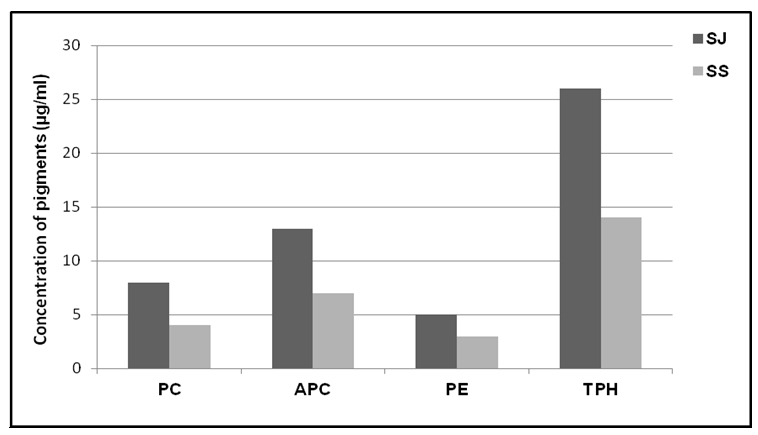
The results of phycobiliprotein content in tested Spirulina strains showed that higher content of all investigated pigments had Spirulina strain SJ (Fig 5). The concentration of PC in Spirulina SJ strain was 8 µg/ml, while Spirulina SS strain had content of 4 µg/ml. The concentrations of APC and PE in the case of strain SJ were 13 µg/ml and 5 µg/ml, respectively. Strain SS had smaller content of both pigments with values of 7 µg/ml for APC and 3 µg/ml for PE (Fig 5). Total pigment content was 26 µg/ml in Spirulina SJ strain, while lower pigment content of 14 µg/ml was found in Spirulina strain SS (Fig 5). <
PC is one of the major pigment constituent of Spirulina, a microalga used in many countries as dietary supplement whose nutritional and therapeutic values have been very well documented (Eriksen, 2008). Because of that Spirulina is one of the economically important cyanobacterial genus. Species of Spirulina have now become the wellknown and the most broadly cultivated microalgae in the world (Vonshak, 1997). Nagaoka et al. (2005) showed that phycocyanin from Spirulina platensis strongly influenced serum cholesterol concentrations and imparted a stronger hypocholesterolemic activity. In this respect Spirulina is one of the most promising microalga. In the present study we tested two strains of Spirulina genera for phycobiliprotein production. Spirulina strain SJ contained higher amount of all three pigments (PC-8 µg/ml, APC-13 µg/ml and PE-5 µg/ml) compared to Spirulina strain SS (PC-4 µg/ml, APC-7 µg/ml and PE-3 µg/ml) (Fig 5). Pigment composition of both strains was very similar. Among phycobilins the highest amount was found for allophycocyanin, then for phycocyanin. The lowest content of phycoerythrin compared to other two types of phycobilins, characterized both strains. This analysis demonstrates that Spirulina strains SJ and SS did not show the highest content of tested phycobiliproteins. Strain SJ had higher content of total phycobiliproteins compared to 50% of other tested strains which belong to Nostoc or Anabaena strains (C5, 2S9B, S7B and 2C1).
Patel et al., (2005) investigated the quantitative of phycobiliprotein (C-PC, APC, and PE) content in three different cyanobacterial species, i.e., Spirulina sp., Phormidium sp., and Lyngbya sp. Among all the three cyanobacterial species, Spirulina sp. contains maximum phycobiliprotein content, i.e., 22.5% (w/w) of total freezedried cell mass, while Phormidium sp. and Lyngbya sp. contain 5.4% (w/w) and 5.8% (w/w), respectively. Based on these results the maximum quantity of C-PC exists in Spirulina sp. (17.5% (w/w)) as compared to Phormidium sp. (4.1% (w/w)) and Lyngbya sp. [3.9% (w/w)], while APC and PE are present at lower quantities (Patel et al., 2005). The results of the present study suggest that some Nostoc and Anabaena strains had much higher content of phycobiliproteins compared to both Spirulina strains SJ and SS.
The obtained results clearly prove the fact that the composition and content of phycobilin pigments are specific characteristics of every individual cyanobacterial strain which is very dependent on growing conditions. The medium composition influences the normal growth of cyanobacteria and normal development of physiological processes. The conditions of cultivation, particularly nitrogen and carbon sources, determines the content of phycobiliproteins in cyanobacteria (cit. in Seker and Chandramohan, 2008). In our study the greatest content of total phycobiliproteins was present in the most of the tested strains which were cultivated in the conditions without nitrogen. Kaushik (2000) obtained similar results by examining the content of phycobiliproteins of 41 strains of cyanobacteria. Results showed that singlecelled and colonial cyanobacteria had the lowest content of phycobiliproteins (less than 2.93% of dry mass), non-heterocistous cyanobacteria also had low level of pigment content, while heterocistous nitrogen fixing cyanobacteria had the greatest production of phycobilin which varied from 14.72 to 17.52% of dry mass. Also, Hemlata (2009) found that Anabaena NCCU-9 produces the largest amount of phycobilins in the condition without nitrogen. Loreto et al. (2003) showed that the strain Anabaena 7120 produces more phycobiliproteins if it is cultivated in the medium without nitrogen, in comparison to the growth in presence of nitrogen. Patel et al. (2005) also noticed the importance of growing conditions on the pigment composition and production of phycobilins for cyanobacterial species. Likewise, Prassana et al. (2004) pointed out the fact that cyanobacteria can regulate their composition and content of basic unit of phycobilin, tetrapyrrole depending on the conditions or signals from the surroundings, such as the availability of nutrients, intensity and quality of light and temperature. In order to find out optimum culture condition for algal growth, Kumar et al. (2011) investigated the effect of light irradiance and temperature on growth rate, biomass composition and pigment production of Spirulina platensis. Maximum contents of phycobiliproteins were found in cultures grown at 35 °C i.e. 7.73 % phycocyanin (PC), 3.46% allophycocyanin (APC) and 1.80 % phycoerythrin (PE) and minimum was observed at 20 °C (5.39 % PC, 2.59% APC and 0.64 % PE). But the phycobiliprotein accumulation (except PE) did not show any significant difference at temperatures 30 °C and 35 °C (Kumar et al., 2011).
The obtained results clearly prove the fact that the composition and content of phycobilin pigments are specific characteristics of every individual cyanobacterial strain which is very dependent on growing conditions. The medium composition influences the normal growth of cyanobacteria and normal development of physiological processes. The conditions of cultivation, particularly nitrogen and carbon sources, determines the content of phycobiliproteins in cyanobacteria (cit. in Seker and Chandramohan, 2008). In our study the greatest content of total phycobiliproteins was present in the most of the tested strains which were cultivated in the conditions without nitrogen. Kaushik (2000) obtained similar results by examining the content of phycobiliproteins of 41 strains of cyanobacteria. Results showed that singlecelled and colonial cyanobacteria had the lowest content of phycobiliproteins (less than 2.93% of dry mass), non-heterocistous cyanobacteria also had low level of pigment content, while heterocistous nitrogen fixing cyanobacteria had the greatest production of phycobilin which varied from 14.72 to 17.52% of dry mass. Also, Hemlata (2009) found that Anabaena NCCU-9 produces the largest amount of phycobilins in the condition without nitrogen. Loreto et al. (2003) showed that the strain Anabaena 7120 produces more phycobiliproteins if it is cultivated in the medium without nitrogen, in comparison to the growth in presence of nitrogen. Patel et al. (2005) also noticed the importance of growing conditions on the pigment composition and production of phycobilins for cyanobacterial species. Likewise, Prassana et al. (2004) pointed out the fact that cyanobacteria can regulate their composition and content of basic unit of phycobilin, tetrapyrrole depending on the conditions or signals from the surroundings, such as the availability of nutrients, intensity and quality of light and temperature. In order to find out optimum culture condition for algal growth, Kumar et al. (2011) investigated the effect of light irradiance and temperature on growth rate, biomass composition and pigment production of Spirulina platensis. Maximum contents of phycobiliproteins were found in cultures grown at 35 °C i.e. 7.73 % phycocyanin (PC), 3.46% allophycocyanin (APC) and 1.80 % phycoerythrin (PE) and minimum was observed at 20 °C (5.39 % PC, 2.59% APC and 0.64 % PE). But the phycobiliprotein accumulation (except PE) did not show any significant difference at temperatures 30 °C and 35 °C (Kumar et al., 2011).
It is significant to point out that the content of phycobiliproteins of examined cyanobacterial strains from Vojvodina region represent the pigment content characteristic for every strain during the stationary phase of growth and in constant and usual conditions of cultivation. There was no additional stimulation of production, and changes of the environmental factors (intensity and quality of light, temperature and concentration of nutriaents) could influence pigment production in all strains and combining appropriate conditions a high amount of phycobiliproteins can be synthesized. Improvement in the phycobilin content with changes of environmental factors such as light intensity and quality could be a good basis for the exploitation of studied microalgae as a source of biopigments. Still, it is possible that the production of phycobilins would have been increased if other factors had been used, which requires an additional research.
CONCLUSION
The pycobiliproteins have good potential and diverse applications. There is increasing interest in the production of these pigments primary because of their food application (for coloring purposes in foods, dairy products, ice creams, soft drinks, beverages and cake icing). The results of the present study showed that some autochtone terrestrial filamentous cyanobacterial strains from Vojvodina region had good potential for phycobiliprotein production.
Strains such as Anabaena C2, Anabaena LC1B and Nostoc S1 are very promising producers since they showed the highest content of all three phycobiliproteins among other tested strains. In that respect, it is significant to point out that these strains had much higher pycobiliprotein content compared to both tested Spirulina strains.
Further investigations are needed to improve their productivity in suit conditions in mass cultivation. Also, their toxicity should be investigated.
ACKNOWLEDGMENTS
This study has been supported by the funding of the Ministry of Education and Science of the Serbian Government (project number: TR 31029) which is greatly acknowledged.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE