THE INFLUENCE OF BONSILAGE FORTE ON FERMENTATION AND AEROBIC STABILITY DURING ALFALFA ENSILING
Bulevar cara Lazara 1, Serbia
ABSTRACT
ABSTRACT
INTRODUCTION
Legumes are difficult to ensile successfully without an additive (Carpintero et al., 1969; Ohshima et al., 1979). This is espe-cially true for alfalfa (Medicago sativa L.). McDonald et al. (1991) considered that the difficulties in ensiling legumes were attri-butable to three factors. Firstly, they are highly buffered; secondly, they tend to have low water-soluble carbohydrate (WSC) contents; and thirdly, they often have low dry-matter (DM) content.
The number of LAB present on alfalfa plants at harvest may be too low to ensure rapid and efficient preservation, and the-refore silage inoculants have been deve-loped to improve the nutritive value of si-lages and to reduce risks during ensiling (Čabarkapa, 2010a).
The possible beneficial effect of LAB ino-culants depends on their composition, concentration and the properties of the crop being ensiled (Tengerdy et al., 1991).
Regarding the effect of bacterial inoculant, some authors (Weinberg et al., 1988; Ten-gerdy et al., 1991; Masuko et al., 2002) indicated that inoculation of the alfalfa si-lages with LAB enhanced the silage qua-lity, while others reported no effect (Lind-gren et al., 1983; Ohyama et al., 1973). The aim of this study was to determine effects of adding a silage inoculant with a new combination of LAB strains to alfalfa, grown under south-eastern European conditions, on fermentation dynamics and aerobic stability of silage.
MATERIAL AND METHODS
The alfalfa (280.6 g/kgDM) was compact-ted into 1.8 dm3 transparent containers-with a special water valve to enable gas release, as proposed by Palić etal. (2011). Each container was filled with approximately 0.65 kg (wet mass) alfalfa without a headspace, and a packing density of approximately 360 kg/m3 was obtained.
A total of 12 containers were divided into two groups. Each group consisted of three control containers, without inoculant, and three inoculated samples, with Bonsilage Forte added. Containers were stored in dark roomat a temperature of 21-25 0C.
On day 0, fresh alfalfa was collected for subsequent chemical analysis. First group was opened on day 14 and second on day 49 day for determination of pH, dry matter (DM), crude protein (CP), ammonia nitro-gen (NH3-N), lactic acid (LA) and volatile fatty acids (VFA). The pH, DM and CP were determined following the procedures of AOAC (2005). The NH3-N, LA acid and VFA were determined according to stan-dard methods (Die Chemische Untersu-chung von Futtermitteln, 1997). On day 49 silages were also subjected to an aerobic stability test conducted according to pro-cedure of Ashbell et al. (1991).
Statistical Analysis System STATISTICA (2011) was used for analyzing variations (ANOVA) and least significant differences. The level of significance was set at p<0.05.
Results and Discussion
Initial pH value of alfalfa (6.43) decreased to a greater extent in inoculated samples and was about 5.0 on day 49, compared to control samples where it was slightly be-low 6.0 (Figure 1). Also, pH drop of inocu-lated silage in the first days of ensiling was faster, which is important for conserving of nutrients of the silage by inhibiting spoil-age microorganisms (present in the natu-ral plant microflora) and hetrofermentative bacteria (enterobacteria) and promoting homofermentative lactic-cid bacteria. Va-lue of pH is a key criterion to evaluate silage fermentation. Generally, the lower the pH, the better preserved and more stabile is the silage (Seglar, 2003).
Fermentation parameters of experimental silages (lactic acid, acetic acid, butyric acid and ammonia nitrogen concentration) are shown in the table 2. Concentration of lactic acid was significantly higher in ino-culated samples and its concentration was doubled on day 49 compared to day 14. Generally, the presence of high lactic acid levels indicates efficient fermentation and minimal dry matter loss (Seglar, 2003).
Analysis/Sample |
Sampling day |
||
|
|
0 |
14 |
49 |
|
DM (g/kg DM) |
|
||
|
280.6 |
|
|
|
|
- |
237.5 ± 8.2 a |
254.9 ± 10.6 a |
|
|
- |
237.5 ± 5.5 a |
246.9 ± 5.6 a |
|
|
CP (g/kg DM) |
|
||
|
63.3 |
|
|
|
|
- |
50.9 ± 2.7 a |
61.8 ± 3.8 a |
|
|
- |
53.2 ± 1.7 a |
61.0 ± 3.2 a |
|
BF - with inoculant
Results are given as mean ± standard deviation
abMeans with different superscripts in the same column are significantly different (P<0.05)
.jpg) Figure 1. pH of fresh alfalfa (day 0) and experimental silages
Figure 1. pH of fresh alfalfa (day 0) and experimental silages (C - without inoculant; BF - with inoculant)
Analysis/Sample |
Sampling day |
||
|
|
0 |
14 |
49 |
|
Lactic acid (g/kg DM) |
|
||
|
2.2 |
|
|
|
|
- |
3.1 ± 0.1 a |
6.6 ± 0.5 a |
|
|
- |
5.1 ± 0.3 b |
10.6 ± 1.6 b |
|
|
Acetic acid (g/kg DM) |
|
||
|
3.1 |
|
|
|
|
- |
8.7 ± 0.4 a |
11.8 ± 1.6 a |
|
|
- |
11.7 ± 0.8 b |
17.6 ± 0.9 b |
|
|
Butyric acid (g/kg DM) |
|
||
|
0.0 |
|
|
|
|
- |
0.04 ± 0.05 a |
0.08 ± 0.9 a |
|
|
- |
0.00 a |
0.00 a |
|
|
NH3-N (g/kg DM) |
|
||
|
0.7 |
|
|
|
|
- |
1.7 ± 0.0 a |
2.1 ± 0.1 a |
|
|
- |
1.6 ± 0.1 b |
1.8 ± 0.0 b |
|
BF - with inoculants
Results are given as mean ± standard deviation
abMeans with different superscripts in the same column are significantly different (P<0.05)
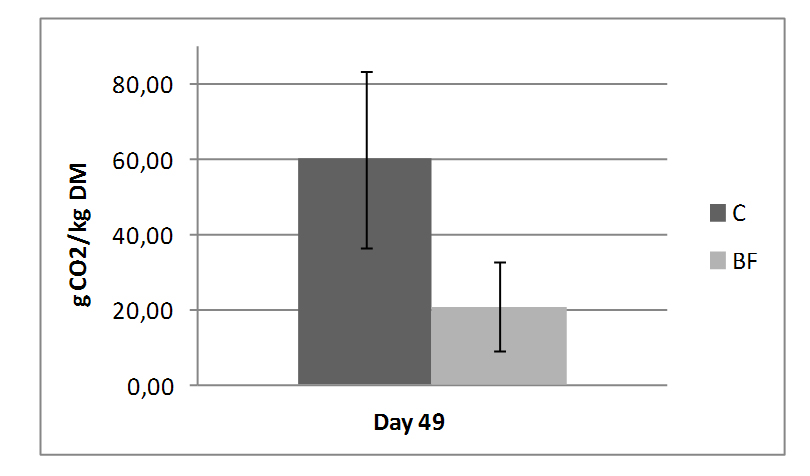 Figure 2. Aerobic stability of experimental silages on day 87(C - without inoculant; BF-with inoculant) Results are given as mean ± standard deviation
Figure 2. Aerobic stability of experimental silages on day 87(C - without inoculant; BF-with inoculant) Results are given as mean ± standard deviationPresence of butyric acid is the result of Clostridial activity (Seglar, 2003). Butyric acid was detected only in control samples. Clostridia can grow at lower pH values than enterobacteria making them more di-fficult to control (Čabarkapa et al. 2010b). If pH value cannot be decreased deep enough the Clostridia spores germinate and degrade lactic acid to butyric acid.
Concentration of ammonia nitrogen (NH3-N) increased through the expe-riment, but it was significantly lower in ino-culated samples. Higher ammonia indic-ates protein brake down from proteolytic enzymatic activity (Seglar, 2003).
Results of aerobic stability test on day 49 (Figure 2), expressed by released CO2 upon exposure to air, showed positive effect of inoculants on aerobic stability of silage. Amount of released CO2 was three times lower in inoculated samples com-pared to control.
Conclusions
Lower pH in inoculated samples probably inhibited protein degradation and therefore concentrations of ammonia-nitrogen were lower in those samples, which demonstra-tes positive effect of Bonsilage Forte on nutritive value of silage.
Addition of inoculants resulted in a signify-cantly higher lactic concentration than in the control samples. Lactic acid is the strongest of all silage acids and its pre-sence drops pH more effectively than other volatile fatty acids.
Absence of butyric acid in inoculated samples confirms that Clostridial second-dary fermentation did not take place and that Bonsilage Forte acted efficiently against Clostridia.
The aerobic stability of silage, as measu-red by the release of CO2 after exposure to air, was significantly improved by addi-tion of BF.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE