POTENTIAL OF BIOACTIVE PROTEINS AND PEPTIDES FOR PREVENTION AND TREATMENT OF MASS NON-COMMUNICABLE DISEASES
Miona M. Belović*1, Jasna S. Mastilović1, Aleksandra M. Torbica1, Jelena M. Tomić1, Dušica R. Stanić3, Natalija R. Džinić2
2University of Novi Sad, Faculty of Technology, 21000 Novi Sad, Bulevar cara Lazara 1, Serbia
3University of Istočno Sarajevo, Faculty of Technology-Zvornik, 75400 Zvornik, Karakaj b.b. mjesto,
Bosnia and Herzegovina
ABSTRACT
Abstract
Introduction
Bioactive peptides can be classified on the basis of their origin as animal origin pep-tides and plant origin peptides. Animal sources include dairy products, egg, meat, insects, fish and seafood (Wang et al., 2005). Plant sources usually include ce-reals, such as wheat, corn, rice, barley, rye and pseudocereals, such as buck-wheat and amaranth. Other plant sources are legumes (soy, pea, and chickpea), Brassicaceae species (mustard, rapeseed) and other (sunflower). Among plant sour-ces, soybean is the most studied source of bioactive proteins and peptides. This can be explained by the fact that soybean is an important protein source. On average, soybean contains about 40% protein (Wang et al., 2005). In Western countries, other mentioned cultures are grown more than soy and they should be further studied for their biological activity.
BIOLOGICAL FUNCTIONS OF BIOACTIVE PEPTIDES
Anticancer
factor in the developed world (Maldonado et al., 2010). Carcinogenesis is a process that consists of several steps. Many differ-rent factors, both genetic and environ-mental, affect initiation and promotion of cancer (Ledesma et al., 2009). Chemical carcinogenesis and viral oncogenesis have been found to share common me-chanisms involving changes in chromatin status. Histone acetylation and deace-tylation are involved in chromatin remo-deling, which is important in cell cycle con-trol (Ledesma et al., 2009). Anticarci-nogenic peptides have no common me-chanism of action, but they eventually lead to the inhibition of mitosis and cell death.
Investigations of anticancer activity of pep-tides derived from plant proteins are pre-sented in Table 1. It can be seen that most researchers have investigated anticancer activity of lunasin.
Lunasin is firstly discovered in soy (Her-nández-Ledesma et al., 2009), and later in some cereals and pseudocereals, like wheat, barley, rye, rice and amaranth. It consists of 43 amino acid residues. The antimitotic activity of lunasin is explained by 8 Asp residues at carboxyl end of its chain which are able to bind to the hypoacetylated regions of chromatin (Her-nández-Ledesma et al., 2009).
Disease/condition |
Biological assay |
Encoding protein/protein hydrolysate |
Source |
References |
|
Cancer |
In vitro and in vivo |
Lunasin |
Wheat |
Jeong et al., 2007 |
|
Cancer |
In vitro |
Lunasin |
Barley |
Jeong et al., 2002 |
|
Cancer |
In vitro and in vivo |
Lunasin |
Rye |
Jeong et al., 2009 |
|
Cancer |
In vitro |
Lunasin from glutelin fraction |
Amaranth |
Silva-Sánchez et al., 2008; Maldonado-Cervantes et al., 2010 |
|
Cancer |
In vitro and in vivo |
Lunasin |
Soy |
Jeong et al., 2003, 2007; De Mejia et al., 2003, 2004; Park et al., 2005; Hernández-Ledesma et al., 2009 |
|
Cancer |
In vitro |
Pentapeptide |
Rice |
Kannan et al., 2010 |
|
Cancer |
In vitro and in vivo |
Bowman-Birk inhibitor |
Soy |
Park et al., 2005 |
|
Cancer |
In vitro and in vivo |
Lectins |
Soy |
De Mejia et al., 2003; Barać et al., 2005 |
Kannan et al. (2010) isolated and charac-terized novel anticancer pentapeptide de-rived from rice bran enzymatic hydro-lysate, whose amino acid sequence is Glu-Gln-Arg-Pro-Arg. This peptide is resistant to gastrointestinal juices and possesses cancer growth inhibitory properties on colon, breast, lung and liver cancer cells.
One of the most extensively studied bio-active substances in soy is the Bowman-Birk protease inhibitor (BBI) (Jeong et al., 2003; Park et al., 2005; Hernández-Le-desma et al., 2009). BBI is a serine protease inhibitor that consists of a single chain of 71 amino acid residues. This chain is cross-linked by seven pairs of disulfide bonds. It has the ability to inhibit the action of trypsin and chymotrypsin (Park et al., 2005, Barać et al., 2005). BBIC (BBIC refers to BBI concentrate, crude form) has been shown to be cancer preventive in vitro models of carcino-genesis. In preclinical studies, BBIC has been found to interfere with the develop-ment of tumors induced by chemical carcinogens in a number of animal model systems (Park et al., 2005).
Lectins are glycoproteins that can selec-tively bind carbohydrates (Mejia et al., 2003). Lectins show several biochemical, physiological and nutritional effects after ingestion. Some of them are agglutination of red blood cells and stimulation of pan-creatic enzyme secretion, resulting in re-duced intestinal absorption of nutrients. There are several reports that plant lectins may have antitumor and anticar-cinogenic activities that could be useful in cancer treatment. The exact mechanism of the antitumor effect of plant lectins is not clear, although several mechanisms have been proposed. Some of these mechanisms are reduction of cell division, increasing the number of macrophages, increasing the susceptibility of tumor cells to macrophage attack and serving as a bridge between tumor cells and macrophages (Barać et al., 2005).
Regulation of the immune system
Effect of peptides on the immune system has been the topic of several researches, which are presented in Table 2. It is usually studied among other functiona-lities, but Dia et al. (2009) reported anti-inflammatory activity of lunasin. It pre-vents inflammation by inhibiting COX-2/PGE2 and iNOS/NO pathways. Previous investigations of lunasin were focused on its cancer preventive activity. However, inflammation is a critical factor in tumor progression and cells that suffer infla-mmation produce various responses that can damage DNA and cause mutations that lead to tumor initiation and/or pro-motion. Horiguchi et al. (2005) reported that intake of wheat gluten hydrolysate can increase NK (natural killer) cell activity without severe side effects. NK cells play a major role in the rejection of tumors and cells infected by viruses. Oryzatensin, an ileum contracting bioactive peptide ob-tained from rice albumin, was shown to have immunostimulatory role that was me-diated by histamine release. Its amino acid sequence is Gly-Tyr-Pro-Met-Tyr-Pro-Leu-Pro-Arg (Takahashi et al., 1996 in Kannan et al., 2010).
Disease/condition |
|
Biological assay |
Encoding protein/protein hydrolysate |
Source |
References |
|
Immune system |
Inflammation |
In vitro |
Lunasin |
Soy |
Dia et al., 2009 |
|
Immune system |
Immunomodulation |
In vivo |
Wheat gluten hydrolysate |
Wheat |
Horiguchi et al., 2005 |
|
Immune system |
Immunomodulation |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Immune system |
Immunomodulation |
N.A. |
Oryzatensin from rice albumin |
Rice |
Takahashi et al., 1996 in Kannan et al., 2010 |
|
Immune system |
Immunostimulating |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
Regulation of the cardiovascular system and metabolic disorders
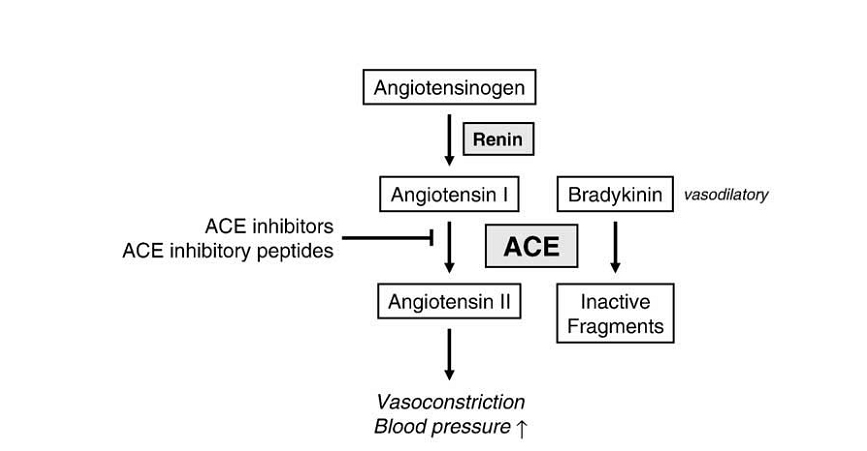
High blood pressure is one of the major risk factors for CVD (Erdmann et al., 2008). Angiotensin I-converting enzyme (ACE, EC 3.4.15.1) is one of the main regulators of blood pressure because it acts on two body systems. Firstly, ACE forms part of the renin–angiotensin system (RAS), converting angiotensin I to angio-tensin II, a potent vasoconstrictor. Angio-tensin II also induces the release of aldosterone and therefore increases the concentration of sodium in circulation and blood pressure. Secondly, ACE hydrolyzes bradykinin, which has a vasodilatory effect and forms part of kinin-kallikrein system. Different synthetic ACE inhibitors, such as Captopril and Enalapril, are being used worldwide to treat hypertension (Hernán-dez-Ledesma et al., 2011). The reninan-giotensin system is presented in Figure 1.
Numerous peptides with beneficial effects on the cardiovascular system and related metabolic disorders have been discovered up to date. However, most of protein hy-drolysates and identified peptides have hypotensive effect and they are presented in Table 3.
All these peptides have common mecha-nism of action - inhibition of angiotensin I-converting enzyme. It is supposed that peptides that consist of two or three amino acid residues could be absorbed directly from the gastrointestinal tract into the blood circulation. These intact peptides are able to exert physiological action (Wu et al., 2006). Peptides with Pro or hydroxy-Pro as C-terminus are usually resistant to degradation by digestive enzymes (Erd-mann et al., 2008).
Disease/condition |
|
Biological test |
Encoding protein/protein hydrolysate |
Source |
References |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Wheat germ protein hydrolysate |
Wheat |
Matsui et al.; 1999, Jia et al., 2010 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro and in vivo |
Gliadin hydrolysate |
Wheat |
Motoi et al., 2003 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
α-Zein hydrolysate |
Corn |
Yano et al., 1996 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
Database |
Peptides from globulin 11S |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Protein hydrolysate |
Pea |
Humiski and Aluko, 2007; Li and Aluko, 2010 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Legumin hydrolysate |
Chickpea |
Yust et al., 2002 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Protein hydrolysate |
Mustard |
Pedroche et al., 2007 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Helianthinin hydrolysate |
Sunflower |
Megias et al., 2004 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro, prediction |
Peptides from glycinin |
Soy |
Wu et al., 2006 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro, prediction |
Peptides from β-conglycinin |
Soy |
Wu et al., 2006; Chen et al., 1995 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro, prediction |
Peptides from legumin |
Pea |
Wu et al., 2006 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro, prediction |
Peptides from albumin |
Pea |
Wu et al., 2006 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro, prediction |
Peptides from vicilin |
Pea |
Wu et al., 2006 |
|
Cardiovascular system |
Hypertension (ACE inhibitor) |
In vitro |
Protein isolate |
Rapeseed |
Yoshie-Stark et al. 2006, 2008 |
Hydrolysates from legumes, pea and chickpea, also contain peptides with hypo-tensive effect. Li and Aluko (2010) reported three ACE inhibitory peptides from pea protein hydrolysate, whose se-quences were Ile-Arg, Lys-Phe and Glu-Phe. Antihypertensive peptides isolated from chickpea legumin hydrolysate consist mostly of Met, Asp, Phe and Leu (Yust et al., 2002).
Other industrial cultures were also invest-tigated for peptides with ACE inhibitory activity. Fractions obtained from Brassica carinata (Ethiopian mustard) protein hy-drolysate showed ACE inhibitory activity (Pedroche et al., 2007). Megias et al. (2004) obtained an ACE inhibitory peptide with the sequence Phe-Val-Asn-Pro-Gln-Ala-Gly-Ser from helianthinin, the 11S glo-bulin from sunflower seeds, which is the main storage protein in sunflower.
Some of bioactive peptides have not been discovered yet, but there are indications that they could be obtained by hydrolysis of natural proteins. Wu et al. (2006) con-structed from published literature a data-base consisting of 168 dipeptides and 140 tripeptides to study the quantitative struc-ture-activity relationships of angiotensin I-converting enzyme (ACE) inhibitory pep-tides. Three predicted dipeptides and four predicted tripeptides were located within the primary structure of food proteins. Then they were synthesized for validation of their IC50 values through laboratory determination of inhibition of ACE activity. Three peptides determined in their study were more potent than the well-known milk tripeptides of Val-Pro-Pro and Ile-Pro-Pro. Their sequences were Leu-Arg-Trp, Ile-Lys-Pro and Phe-Trp. Leu-Arg-Trp and Phe-Trp were located in pea protein se-quence, and Ile-Lys-Pro was located in soybean and pea proteins.
Yoshie-Stark et al. (2008) found that pro-tein isolate from rapeseed might be a good source of ACE inhibitory peptides. The highest ACE inhibition was shown by pepsin-pancreatin digested precipitated protein isolate.
Oxidative stress, the increased production of reactive oxygen species (ROS), is another significant factor for the initiation or progression of several vascular disea-ses. ROS can cause extensive damage to biological macromolecules like DNA, pro-teins and lipids. Moreover, the oxidation of LDL leads to the increasing of its athe-rogenicity (Erdmann et al., 2008). Several amino acids, such as Tyr, Met, His, Lys, and Trp, are generally accepted to be antioxidants (Wang et al., 2005). High amounts of His and hydrophobic amino acids in peptides contribute to their antio-xidant activity. Among all tested peptides, those with a Pro-His-His sequence sho-wed the greatest antioxidant activity (Erdmann et al., 2008). Wheat germ and wheat gluten hydrolysates showed in vitro antioxidative and free-radical scavanging action. Defatted wheat germ protein iso-lates were hydrolyzed using alcalase. Biological activity of obtained wheat germ protein hydrolysates was determined by several tests: antioxidative activity in linoleic acid emulsion system, scavenging effect on DPPH free radical, superoxide radical-scavenging activity, hydroxyl ra-dical-scavenging activity, reducing power and ferrous ion-chelating activity (Zhu et al., 2006). Enzymatic hydrolysis was also used for preparing hydrolysates from wheat gluten and the enzyme used for the hydrolysis was papain. Antioxidative ac-tivity was measured using DPPH radical and TBA method (Wang et al., 2007). Iwami et al. (1987) showed that wheat gliadin, obtained by extraction of wheat gluten with 70% ethanol, has the most potent antioxidative action against pero-xidation of linoleic acid. Other cultures whose protein hydrolysates showed antio-xidative and free-radical scavanging acti-vity are amaranth, pea and mustard.
Antioxidative activity of pea protein hydro-lysate was confirmed by DPPH radical scavanging activity (Humiski and Aluko, 2007; Pownall et al., 2010), superoxide scavenging activity, hydrogen peroxide (H2O2) scavenging activity, hydroxyl ra-dical (OH•) scavenging assay, reducing power, metal chelating assay and inhibi-tion of linoleic acid oxidation. The peptide fractions from pea had greater ability to inhibit linoleic acid oxidation and chelate metals than glutathione. However, they showed lesser ability to scavenge free ra-dicals in comparison with glutathione (Pownall et al., 2010). Peptide fractions obtained by hydrolysis of Brassica carinata protein also showed antioxidative activity. It was confirmed by the antioxidant assay that used the discoloration of β-carotene, because β-carotene is extremely suscep-tible to oxi-dation caused by free radicals (Pedroche et al., 2007).
Table 3.
Examples of peptides derived from plant proteins with other effects on the cardiovascular system and metabolic disorders
Disease/condition |
|
Biological test |
Encoding protein/protein hydrolysate |
Source |
References |
|
Cardiovascular system |
Oxidation |
In vitro |
Wheat germ protein hydrolysate |
Wheat |
Zhu et al., 2006 |
|
Cardiovascular system |
Oxidation |
In vitro |
Wheat gluten hydrolysate |
Wheat |
Wang et al., 2007 |
|
Cardiovascular system |
Oxidation |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Cardiovascular system |
Oxidation |
In vitro |
Protein hydrolysate |
Pea |
Humiski and Aluko, 2007; Pownall at al., 2010 |
|
Cardiovascular system |
Oxidation |
In vitro |
Protein hydrolysate |
Mustard |
Pedroche et al., 2007 |
|
Cardiovascular system |
Oxidation |
In vitro |
Wheat gliadin |
Wheat |
Iwami et al., 1987 |
|
Cardiovascular system |
Oxidation |
In vitro |
Protein isolate |
Rapeseed |
Yoshie-Stark et al. 2006, 2008 |
|
Cardiovascular system |
Thrombosis |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Cardiovascular system |
Hyperlipidemia |
In vitro |
Protein hydrolysate |
Mustard |
Pedroche et al., 2007 |
|
Cardiovascular system |
Hyperlipidemia |
In vitro and in vivo |
Peptides from globulin |
Soy |
Duranti et al., 2004, Pak et al., 2005 |
|
Cardiovascular system |
Hyperlipidemia |
In vitro |
Proteins from wheat flour |
Wheat |
Tani et al., 1994 |
|
Cardiovascular system |
Hyperlipidemia |
In vitro |
Wheat germ proteins |
Wheat |
Borel et al., 1989 in Möller et al., 2008 |
|
Gastrointestinal system |
Hyperlipidemia |
In vitro |
Protein isolate |
Rapeseed |
Yoshie-Stark, 2006, 2008 |
|
Cardiovascular system |
Diabetes |
In vitro and in vivo |
Wheat albumin |
Wheat |
Kodama et al., 2005 |
|
Gastrointestinal system |
Increased appetite/obesity |
In vivo |
β-Conglycinin fragment |
Soy |
Nishi et al., 2003 in Erdmann et al., 2008 |
Another complication related to CVD is the developing of thrombosis due to disorders in blood coagulation (Erdmann et al., 2008). Peptides with antithrombotic effect were reported by Silva-Sánchez et al., 2008. They were identified in a tryptic digest of amaranth glutelin fraction and their sequences were Pro-Pro-Gly, Pro-Gly, and Gly-Pro.
An unfavorable profile of blood lipids is another major risk factor for the develop-ment of CVD. Many studies have found a positive correlation between hypercho-lesterolemia and/or hypertriglyceridemia and the probability for developing CVD. Peptides with hypocholesterolemic effect have high amounts of hydrophobic amino acids, which enable them to bind bile acids and thereby enhance the excretion of steroids through feces (Erdmann et al., 2008).
Because cholesterol is a water-insoluble molecule, its intestinal absorption has a mechanism similar to that of triglycerides. Cholesterol, as well as triglycerides, re-quires micellar solubilization with bile salts. It has been reported that some peptides can decrease cholesterol solubility by replacing it in micellar structure. Similar hypocholesterolemic peptides were pre-sent in B. carinata protein hydrolysates (Pedroche et al., 2007).
Duranti et al. (2004) demonstrated that α-subunit from soybean 7S globulin exerts both lipid lowering effect in plasma and upregulation of the β-VLDL receptors in liver cells from hypercholesterolemic rats. This polypeptide was given to rats orally. Another study of soybean globulins sho-wed that one peptide, isolated by HPLC from the pepsin hydrolysate of 11S-globulin, also possessed hypocholeste-rolemic activity. Its molecular weight was 755.2 Da and amino acid sequence was Ile-Ala-Val-Pro-Gly-Glu-Val-Ala. The hy-po-cholesterolemic effect of this peptide was determined by two tests. The first test was the analysis of bile acid binding and the second was percent inhibition of 3-hy-droxy-3-methylglutaryl coenzyme A reduc-tase in vitro. The lowering of the cho-lesterol level was explained by the binding of this peptide to the bile acids, which prevented their reabsorption in the gastro-intestinal tract (Pak et al., 2005).
Wheat proteins exhibited hypolipidemic effects in several studies. Tani et al. (1994) isolated proteinous lipase inhibitor (LI) from wheat flour. Molecular masses of the LIs were determined by SDS-PAGE and their values were approximately 28 and 25 kDa. Lipase inhibitory activity was determined by the inhibition of porcine pancreatic lipase. Proteins isolated from wheat germ, whose molecular masses were 24.4 and 27.5 kDa also showed inhibitory effect on lipolysis (Borel et al., 1989 in Möller et al., 2008).
Yoshie-Stark et al. (2008) examined bile acid-binding capacity of rapeseed protein isolates. They found that precipitated pro-tein isolate had higher binding capacity than ultrafiltered protein isolate. It was ex-plained by the higher concentration of fiber in precipitate.
Peptides that have an effect on high level of glucose in blood are usually inhibitors of digestive enzymes that are involved in the degradation of starch.
Objective of research conducted by Ko-dama et al. (2005) was to examine the effects of single dose and long-term administration of wheat albumin (WA) on levels of glucose in blood. WA showed 150 times higher in vitro α-amylase inhi-bitory activity that that of wheat flour. In vivo test was also conducted with 12 heal-thy adult male volunteers for the single administration and with 24 patients with mild type II diabetes for the long-term administration. It was concluded that WA suppressed postprandial hyperglycemia without adverse effects such as hypo-gly-cemia, gastrointestinal symptoms, or he-patotoxicity.
In many developed countries obesity is one of the risk factors for cardiovascular diseases. Hyperinsulinemia, insulin resis-tance and dyslipidemia have been linked to obesity. Lipoprotein profile of obese subjects shows higher levels of triglyce-rides, high LDL-cholesterol and low HDL-cholesterol (Erdmann et al., 2008).
Disease/condition |
|
Biological test |
Encoding protein/protein hydrolysate |
Source |
References |
|
Nervous system |
Opioid agonist |
Database |
Glutelin tryptic digest |
Amaranth |
Silva-Sánchez et al., 2008 |
|
Nervous system |
Opioid agonist |
In vivo |
Gluten exorphins |
Wheat |
Takahashi et al., 2000; Fukudome et al., 1993 in Hartmann and Meisel, 2007 |
Regulation of the nervous system
Food-derived peptides with opioid activity were termed “exorphins” on the basis of their structural similarity to endogenous opioid peptides endorphins. The amino acid sequence common to both endoge-nous and exogenous peptides is an N-terminal tyrosine residue and the presence of another aromatic residue in the third or fourth position from the N terminus (Phe or Tyr). Fukudome et al. (1993) discovered novel opioid peptide exorphin C (Tyr-Pro-Ile-Ser-Leu) in wheat gluten. Takahashi et al. (2000) reported that newly isolated bio-active food protein fragment, gluten exo-rphin A5 (Gly-Tyr-Tyr-Pro-Thr), exhibited opioid activity in mice (Hartmann and Meisel, 2007). Silva-Sánchez et al. (2008) identified several peptides in a tryptic di-gest of amaranth glutelin fraction that can act as potent analgesics.

 JOURNAL TOOLS
JOURNAL TOOLS


 INSTITUTE
INSTITUTE