ABSTRACT
ABSTRACT:
The essential oil of oregano (Origanum heracleoticum L.) was analyzed and the presence and relative ratio of 33 compounds was successfully determined using a retention time locked gas chromatographic method. Results show that concentration of the injected essential oil plays a very important role in accurate determination of the compounds. While compounds which comprise a small ratio of the total oil (less than 1%) can only be successfully determined when a relatively high amount of oil is injected, higher concentrations can lead to shift in retention times and less efficient separation of the compounds. Lower essential oil concentrations yield better peak shapes and more efficient separation of components, but trace compounds present in a small amount may not be detected or identified with sufficient certainty in these cases. Identification of essential oil components based solely on their chromatographic data and MS spectra is not reliable method of their determination, since essential oils represent very complex mixtures of sometimes highly similar isomeric compounds with similar mass spectra. Retention time locking, which uses matching of spectral data with the locked retention times in a corresponding compound database can be helpful during such analyses, but there is a need for inclusion of much greater number of compounds in such databases. For this reason, calculation of Kovats indices still represents an important step in identification of similar compounds in highly complex natural products such as essential oils.
INTRODUCTION
Oregano (Origanum heracleoticum L.) is a plant native to the Mediterranean region which has been used in traditional medicine and as spice in food for centuries. Its spice value and properties are due to the aromatic and other volatile compounds generally referred to as essential oils of the aromatic plant. Essential oils derived from oregano have valuable pharmacological properties that have been investigated by many scientists around the world (Daferera et al., 2003). Due to their antimicrobial, insecticidal, antifungal, and antibacterial activeties, essential oils have been intensely screened and applied in the fields of pharmacology, medical and clinical micro-biology, phytopathology and food presservation (Daferera et al., 2000). Certain essential oils have also been shown to posess cytotoxic effects (Sivropoulou et al., 1996; Bakkali et al., 2008).
Gas chromatography-mass spectrometry (GC−MS) is the most popular method for the determination of essential oil composition. Components existing in the essential oil can be identified by comparison of their relative retention indices and their mass spectra (MS). Identification of individual components of essential oils, however, is not always possible using MS data alone. Often different spectra are reported in a library for a single compound, with different common names, or systematic name, corresponding to an individual component sometimes apparent (Sheille et al., 2002). The spectral similarity of a great number of essential oil components causes difficulty in obtaining positive identification of individual components; mass spectra for sesquiter-penes are often identical or nearly identical (Konig et al., 1999). Some authors have also evaluated different techniques for essential oil analysis, like the more comprehensive two-dimensional gas chromatography (GC×GC) (Dimandja et al., 2000; Sheille et al., 2002). However, GC-MS analysis is still the most widely used method for routine analysis of essential oils, and care must be taken to optimize the chromatographic conditions in order to obtain the most accurate results.
The aim of this work was to evaluate the GC-MS method (Agilent application 5988-6530EN, 2010) for the analysis of oregano essential oil and to examine whether calculation of Kovats indices is still a necessary step in identification of the oil components when using a retention time locked chromatographic method. We have also compared the results of analysis of two different concentrations of the oil to optimize the amount of the injected analyte which would obtain the largest number of the identified compounds without the loss of chromatographic resolution.
MATERIALS AND METHODS
Plant materials and chemicals:
n-Heptane of chromatographic grade and was purchased from Merck (Darmstadt, Germany). A mixture of n-alkanes from n-octane (C8) to eicosane (C20) was used for calculation of Kovats indices (KI). Dried and ground plant material of oregano (Origanum heracleoticum L.) was obtained from the Institute for Medicinal Plant Research “Dr Josif Pančić“ (Belgrade, Serbia).
Isolation of essential oil:
Plant material (50 g of oregano) was subjected to hydrodistillation for 3 h, using a modified Clevenger-type apparatus to produce essential oil. The oil was dried by anhydrous sodium-sulphate (Na2SO4) and kept sealed in dark glass vial at +8 oC until use. Diluted essential oil (1/50 in n-heptane, v/v) was used for GC-MS analyses.
Gas chromatography-mass spectrometry (GC-MS):
GC-MS analyses were carried out using Agilent 5975C Series GC-MSD system (7890A GC and 5975C inert MSD) operating in the EI mode at 70 eV, equipped with a HP-5MS capillary column (30 m × 0.25 mm; film thickness 0.50 μm).
Screener method (Agilent application 5988-6530EN, 2010) using freely available Flavor2 screener compound database for retention time locking (RTL) to n-pentadecane (at 27.500 min) was used for analysis. 1 µl of diluted essential oil was injected in split mode at two different split ratios (25:1 and 12.5:1), and inlet temperature was held at 250 °C. Helium was used as carrier gas in constant pressure mode at 9.4 psi. The oven temperature was programmed as follows: 60 °C raised to 240 °C (3 °C/min) and not held. MSD was operated in scan mode in 40-400 m/z range, with ion source and transfer line temperatures held at 230 and 300 oC, respectively.
Identification of the compounds:
The identification of the compounds was based on comparison of their Kovats indices (KI), their retention times (RT) and mass spectra with NIST/Flavor2 /Adams libraries spectra and literature (Adams, 1995). ChemStation software (Agilent Technologies) was used for data analysis, and curves used for experimental estimation of Kovats indices were plotted and drawn using SciDaVis (http://scidavis.sourceforge.net/) software.
RESULTS AND DISCUSSION
The results of analysis of oregano essential oil (Table 1.) show that a total of 33 compounds can be determined in oregano oil by the chromatographic method used. Using two split ratios (25:1 and 12.5:1) we achieved formally two different concentrations of injected analytes (dilution of 1/1250 and 1/625, respectively).
Table 1. Results of the analysis of the oregano essential oil
Compound |
RT
|
KIexp
|
KIadams
|
Area % (1:12.5 split ratio)
|
Area % (1:25 split ratio)
|
|
|
|
|
|
|
|
α-pinene
|
5.22
|
937.00
|
937.00
|
0.86
|
0.96
|
|
camphene
|
5.58
|
952.00
|
953.00
|
0.22
|
0.26
|
|
1-octen-3-ol
|
6.32
|
979.00
|
978.00
|
0.42
|
0.45
|
|
3-octanone
|
6.54
|
987.00
|
986.00
|
0.10
|
0.10
|
|
myrcene
|
6.68
|
992.00
|
991.00
|
0.66
|
0.71
|
|
α-phellandrene
|
7.11
|
1006.00
|
1005.00
|
0.10
|
0.11
|
|
δ -3-carene
|
7.30
|
1012.00
|
1011.00
|
0.07
|
0.08
|
|
α-terpinene
|
7.50
|
1019.00
|
1018.00
|
0.86
|
0.94
|
|
p-cymene
|
7.82
|
1028.00
|
1026.00
|
13.59
|
17.03
|
|
limonene
|
7.91
|
1031.00
|
1031.00
|
0.44
|
0.48
|
|
ociemne
|
8.18
|
1040.00
|
1040.00
|
0.11
|
0.11
|
|
γ-terpinene
|
8.93
|
1062.00
|
1062.00
|
1.84
|
1.97
|
|
sabinene hydrate (trans)
|
9.23
|
1069.00
|
1068.00
|
0.04
|
n.d.
|
|
terpinolene
|
10.02
|
1089.00
|
1088.00
|
0.15
|
0.16
|
|
linalool
|
10.44
|
1099.00
|
1098.00
|
0.27
|
0.29
|
|
isoborneol (isomer 2)
|
13.11
|
1168.00
|
1165.00
|
0.34
|
0.52
|
|
4-carvomenthenol
|
13.62
|
1179.00
|
1177.00
|
0.86
|
0.78
|
|
α-terpineol
|
14.16
|
1191.00
|
1189.00
|
0.15
|
n.d.
|
|
dihydro carvone (trans)
|
14.70
|
1203.00
|
1200.00
|
0.10
|
n.d.
|
|
carvacrol methyl ether
|
16.48
|
1246.00
|
1244.00
|
0.62
|
0.61
|
|
carvone
|
17.00
|
1258.00
|
1256*
|
0.11
|
n.d.
|
|
anethole (trans)
|
18.28
|
1287.00
|
1283.00
|
0.07
|
n.d.
|
|
thymol
|
18.74
|
1294.00
|
1290.00
|
7.58
|
7.78
|
|
carvacrol
|
19.51
|
1309.00
|
1305**
|
69.51
|
83.45
|
|
β-caryoplhyllene
|
24.08
|
1419.00
|
1418.00
|
1.27
|
1.28
|
|
linalyl-butirate
|
25.50
|
1453.00
|
1450.00
|
0.19
|
0.20
|
|
γ-muurolene
|
26.50
|
1477.00
|
1477.00
|
0.05
|
n.d.
|
|
β-bisabolene
|
27.87
|
1509.00
|
1509.00
|
1.00
|
n.d.
|
|
γ-cadinene
|
28.06
|
1514.00
|
1513.00
|
0.07
|
0.15
|
|
δ-Cadinene
|
28.45
|
1526.00
|
1524.00
|
0.15
|
n.d.
|
|
spathulenol
|
30.63
|
1578.00
|
1576.00
|
0.04
|
n.d.
|
|
caryophyllene oxide
|
30.83
|
1582.00
|
1581.00
|
0.85
|
0.86
|
|
humulene epoxide II
|
31.87
|
1609.00
|
1606.00
|
0.11
|
0.11
|
* Hognadottir and Rouseff (2003) ** Hennig and Engewald (1994)
Lower concentration of the oil (1:25 split ratio) showed better peak shape and resolution for the separated compounds, but peaks of sabinene hydrate (trans), α-terpineol, dihydro carvone (trans), carvone, anethole (trans), γ-muurolene, γ-cadinene and spathulenol were not present in the chromatogram. Peak of β-bisabolene was present at this concentration, but could not be determined by comparison with mass spectra data-bases with enough certainty for conclusive determination. Peak at 7.92 min was wrongly identified as terpinyl acetate by comparison with mass spectra data-bases at lower concentration, but using the calculated Kovats index of the peak we were able to determine the compound in question as limonene, which is in accordance with the findings of other authors (Adam et al., 1998; Bozin et al., 2006).
Chromatogram of the essential oil at higher concentration is given in the Figure 1. Even at this relatively low concentration of essential oil used (dilution of 1/625), it can be seen that the most abundant peaks (of p-cymene, thymol and carvacrol) show very large areas compared to other compound peaks. Peaks of thymol and carvacrol, which are isomeric compounds, show significant difference between their retention times and are satisfactorily separated, but their calculated Kovats indices slightly differ from the theoretical values. Possible reason for this noted difference between theoretical and experimental values of calculated Ko-vats indices may be a slight shift of retention times of these two compounds due to their relatively high concentrations in the essential oil.
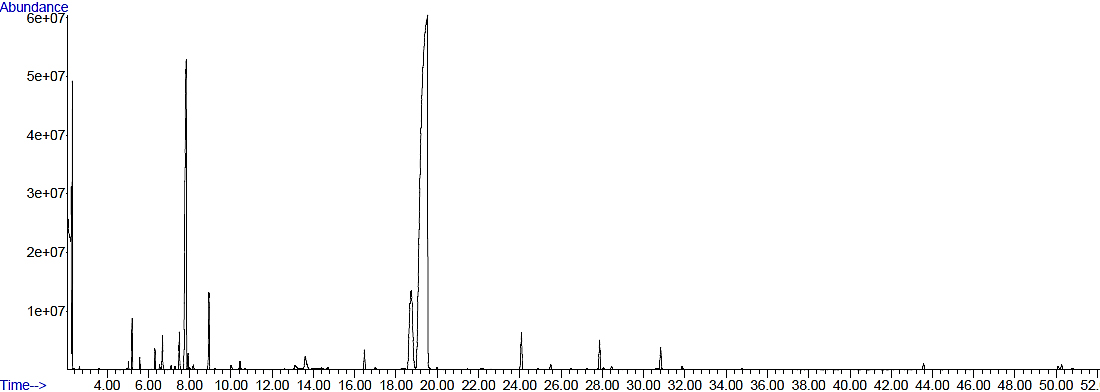 Figure 1. Chromatogram of the oregano essential oil (at 1:12.5 split ratio)
Figure 1. Chromatogram of the oregano essential oil (at 1:12.5 split ratio)
Results show that concentration of the injected essential oil plays a very important role in accurate determination of the compounds. While compounds which comprise a small percent of the total oil (less than 1%) can only be successfully determined when a relatively high amount of oil is injected, higher concentrations can lead to shift in retention times and less efficient separation of the compounds. Lower essential oil concentrations yield better peak shapes and more efficient separation of components, but trace compounds present in a small percentage may not be detected or identified with sufficient certainty in these cases.
The chromatographic method was retention time locked to n-pentadecane in the freely available Flavor2 database, which enabled us to make a screener analysis method, which uses matching of spectral data with the locked retention times in the corresponding screener Flavor2.SCR database. The used method also had a relatively short run time of 60 minutes when compared to some other GC-MS methods used for analysis of essential oils (Mimica-Dukić et al., 2003; Bozin et al., 2006). Chromatogram was analyzed using the screener analysis method, but due to relatively small number of compound entries in the data-base (about 400), only a small number of compounds was determined, and those results were not included in this paper.
CONCLUSION
Referring to the obtained results, screener method (Agilent application 5988-6530EN, 2010) using freely available Flavor2 data-base can be used for the analysis of oregano essential oil composition, but due to the small number of entries in the data-base and shift of retention times in the case of most abundant compounds, calculation of Kovats indices still represents an important step in identification of similar compounds in highly complex natural products such as essential oils.
ACKNOWLEDGEMENTS
Original scientific paper was written as a result of work on a project TR20068 “Prehrambeni proizvodi za grupe potrošača sa specijalnim zahtevima i potrebama“, funded by Ministry of Science and Technological Development, Republic of Serbia.
Download full article PDF
 DOWNLOAD PDF
DOWNLOAD PDF



