Fatty acid composition and natural antioxidant capacity of ten Serbian linseed cultivars
2Institute of Field and Vegetable Crops, Alternative Crops Department,21000 Novi Sad, Serbia
3Biotechnical Faculty, University of Ljubljana,Groblje 3, SI-1230 Domžale, Slovenia
ABSTRACT
Literature data about nutritional characteristics of linseed cultivars from some specific geographical area or country is scarce. For that very reason, following paper is presenting fatty acid (FA) compositions and antioxidant capacity of lipid-soluble (ACL) components of ten native linseed cultivars from Serbia. These characteristics can be interesting, especially due to the increasing trend of linseed usage in human diet. Presented results show that there were statistically significant (p<0.05) differences between linseed kernels in FA composition. Negative correlation was found between FA C18:0 and α-linolenic acid (ALA). The cultivar with the highest ACL value was No. 10 (342.66 μmol trolox/kg d.m.), meaning that it had very strong protection against oxidation of polyunsaturated FAs. Nevertheless, correlation between ACL and polyunsaturated FA content in cultivars was not statistically significant (p=0.84). ACL of the samples did not depend on FA composition of linseed, but it might depend on characteristics of a specific cultivar. The aforementioned results show potential usage in storage of linseeds or its products, while FA composition of linseed kernels might be one of criteria for authentication of linseed origin, and can be of great help in future selection of the cultivars, depending on purpose of linseed production.INTRODUCTION
Not so many commercial crops are so intensively exploited during the history such as linseed. In fact, its Latin name (Linum usitatissimum) means "useful." It was primarily grown (about 3000 B.C.) for medicinal purposes and for the fibres of which were made linen. Nowadays, linseed oil is mainly used, whether it comes to food or chemical industry (Bhatty and Cherdkiatgumchai, 1995). This plant grows to a height up to 60 cm, with slim and very fibrous stems, leaves with three veins, and its light blue flowers. The seeds are rich source of both edible and non-edible oils (Rubilar et al., 2010; Matheson, 1976), containing approximately 40% of oil, of which more than 70% are unsaturated fatty acids (FAs). In addition to a large amount of oil, linseed contains approximately 20-30% of crude protein (Ivanov et al., 2012a; Daun et al., 2003; Karlović and Andrić, 1996).
Raw linseed oil is dark yellow in colour, with strong specific smell and taste (Dimić, 2005). This oil is well known as one of the most unsaturated vegetable oils with high content of ALA, which amount in total fat is more than 50% (Gunstone, 2001). ALA is a precursor of eicosapentaenoic (EPA) and docosahexaenoic acid (DHA), n-3 polyunsaturated FAs, responsible for the proper brain development in children, as well as for resistance to various allergies, autoimmune diseases, cardiovascular problems, and inflammation (Sierra et al., 2008). These FAs are essential, because mammals, and therefore humans, cannot endogenously synthesize them and must adopt them exogenously from dietary sources (Beare - Rogers, 2001). High quality of linseed oil confirms the fact that US National Cancer Institute (NCI) targeted linseed as one of the six plant materials for study as cancer-preventative foods. Linseed oil is a potent inhibitor of proinflammatory mediators even when used in domestic food preparation. This is a great advantage of linseed oil, which make it suitable for application in the development of novel anti-inflammatory therapies with or without pharmaceutical products for target populations (Oomah, 2001).
There are a lot of examples of positive linseed effects on animal health and wellbeing. The immunomodulating effects of omega-3 FAs combined with the potential hormonal effects of the phytoestrogens may have a positive effect on sow productivity and the health of the piglets. Due to the positive impact of including omega-3 FAs in human diets, there is significant interest in enriching the omega-3 FA content of meat products produced by beef cattle and swine. Linseed can be included in poultry diets if used in the proper proportions and formulated appropriately. The most popular commercial poultry product is omega-3 eggs (Newkirk, 2008).
The aim of this study was to determine FA composition and ACL of ten autochthonous linseed cultivars cultivated in the northern Serbian province Vojvodina, in order to detect some mutual characteristics, and to determine whether any significant variation existed in their compositions.
MATERIAL AND METHODS
Material
Examined cultivars of linseeds were developed at the Institute of Field and Vegetable Crops in Novi Sad, Department for Alternative Crops in Bački Petrovac, Serbia. The cultivars were labelled with number from 1 to 10. All of them were cultivated in Vojvodina, the northern Province of Serbia. After harvesting, grains were transported to the laboratory in polypropylene bags and held at room temperature. They were cleaned on an air screen cleaner to remove all foreign matter and impurities such as dust, dirt and immature and broken kernels.
Fatty acid analysis
Supercritical fluid extraction (SFE) with CO2 was used for extraction of lipids from the samples, since it showed good results as a preparative technique for FA analysis. Extraction was performed on LECO TFE‑2000 fat analyzer and extraction conditions were adjusted as explained in the paper of Ivanov et al. (2012b). FA methyl esters were prepared from the extracted lipids by transmetilation method that use 14% wt. boron trifluoride/methanol solution, as recommended method for this type of substrates (Ivanov et al., 2012b; Karlović and Andrić, 1996). Nitrogen gas (99.99%) was used for removing boron trifluoride/methanol solution and n‑heptane (99.99%) from FA methyl esters. Obtained samples were analyzed by a gas chromatograph Agilent 7890A system (Agilent Technologies, Santa Clara, CA, USA) with flame ionization detector (GC-FID), autoinjection module for liquid, equipped with fused silica capillary column (DB‑WAX 30 m, 0.25 mm, 0.50 um). Carrier gas was helium (purity>99.9997 vol %, flow rate=1.26 ml/min). The FAs peaks were identified by comparison of retention times with retention times of standards from Supelco 37 component FA methyl ester mix and with data from internal data library, based on previous experiments and FA methyl ester determination on GC-MS. Results were expressed as mass of single FA or FA group (g) in 100 g of total FAs (relative content).
Antioxidant capacity of lipid-soluble substances analysis
Estimation of the antioxidant capacity in lipid-soluble substances (ACL) was done by photochemiluminescence method using an antioxidant analyser Photochem instrument (Jena Analitik, Germany). In the ACL assay, the photochemical generation of free radicals was measured with a sensitive detector by using chemiluminescence. Free radicals were produced from the luminol, which worked partly as a photosensitizer and partly as an oxygen radical detection reagent. The lipid-soluble antioxidants were measured with the ACL kit, according to manufacturer’s protocol (Jena Analytik, 2004). The working solution consisted of the following reagents:
methanol (Reagent 1) - 2.3 ml,
buffer solution (Reagent 2) - 200 μl,
photosensitizer (Reagent 3) – 25 μl.
Antioxidant capacities of the samples were determined from calibration curve by extrapolation method. Trolox standard with the concentration of 100 μmol/dm3 was used for the preparation of calibration curve. All measurements were done in duplicates.
Statistical analysis
Statistical analysis of experimental data was performed using STATISTICA software version 10 (Statsoft, Tulsa, OK, USA). One way analysis of variance – ANOVA and Tukey’s HSD comparison of means of samples were used for analysing variations. Differences among means with probability p≤0.05 were accepted as representing statistically significant differences. Correlations between FAs and ACL pairs were evaluated at p≤0.05.
RESULTS AND DISCUSSION
FA composition of linseed is well known, however, there are some differences among various cultivars, geographical area, climatic conditions, etc. Table 1 presents FA composition of ten cultivars, examined in this experiment. Obviously, FA composition of linseed oil is dominated by unsaturated C18 FAs (average contents: C18:1 – 22.5%, C18:2 – 14.46%, ALA – 53.23%). Bean and Leeson (2002) reported following mean percentages for these FAs: C18:1 – 18.50%, C18:2 – 14.44% and ALA – 57.11%. Myristic acid (C14:0) was not detected in all samples, and when detected, did not exceed 0.06%. Sauvant et al. (2004) also showed low content of this FA (0.10%), while Bean and Leeson (2002) did not detected it.
Cultivar 1 had significantly (p≤0.05) higher content of saturated FAs (SFA), which accounted for 24.01% of total FAs, in contrast to all other cultivars having from 9.23% to 12.11% of total FAs. Content of monounsaturated FAs (MUFA) ranged from16.44% (cultivar 5) to 22.57% (cultivar 8). Bayrak et al. (2010) reported in their paper average SFA content of 10.02% and MUFA content of 22.47%. Looking at content of polyunsaturated FAs (PUFA), it can be seen that cultivar 1 had significantly lower (p≤0.05) PUFA content 57.79% than all other cultivars, while cultivar number 5 had significantly higher (p≤0.05) PUFA content (74.33%).
|
Linseed cultivar |
|
Fatty acid content (% of total fatty acids) |
|
|||||||||
|
|
C14:0 |
C16:0 |
C16:1 |
C18:0 |
C18:1 |
C18:2 n-6 |
C18:3 n-3 |
SFA |
MUFA |
PUFA |
||
|
1 |
0.00 |
4.58a |
0.20a |
19.43a |
17.99b |
14.83a |
42.97c |
24.01a |
18.20ab |
57.79a |
||
|
2 |
0.00 |
4.89a |
0.08a |
5.96b |
20.77ab |
16.17a |
52.12b |
10.85b |
20.85a |
68.30b |
||
|
3 |
0.00 |
4.93a |
0.07a |
5.82b |
20.30ab |
15.88a |
52.99b |
10.76b |
20.38a |
68.87b |
||
|
4 |
0.00 |
5.31a |
0.08a |
4.08b |
21.03ab |
15.29a |
54.22a |
9.39b |
21.11a |
69.51b |
||
|
5 |
0.00 |
5.42a |
0.11a |
3.81b |
16.33b |
13.27a |
61.06a |
9.23b |
16.44b |
74.33c |
||
|
6 |
0.05a |
5.75a |
0.07a |
4.32b |
20.94ab |
13.34a |
55.52a |
10.13b |
21.06a |
68.86b |
||
|
7 |
0.05a |
6.29a |
0.13a |
5.78b |
21.72ab |
13.16a |
52.87b |
12.11b |
21.91a |
66.03b |
||
|
8 |
0.05a |
5.91a |
0.09a |
4.32b |
22.43a |
14.91a |
52.29b |
10.27b |
22.57a |
67.20b |
||
|
9 |
0.06a |
6.42a |
0.15a |
3.65b |
21.02ab |
13.36a |
55.35a |
10.13b |
21.23a |
68.70b |
||
|
10 |
0.05a |
5.71a |
0.08a |
4.78b |
22.02a |
14.44a |
52.92b |
10.53b |
22.16a |
67.36b |
||
|
Mean |
0.05 |
6.09 |
0.12 |
6.74 |
22.55 |
14.46 |
53.23 |
11.74 |
20.59 |
67.69 |
||
The values are presented as mean, n=3
a-c Different superscripts within the same column indicate significant differences (p≤0.05)
|
|
C14:0 |
C16:0 |
C16:1 |
C18:0 |
C18:1 |
C18:2 |
C18:3 n-3 |
|
C14:0 |
1 |
||||||
|
C16:0 |
0.883* |
1 |
|||||
|
C16:1 |
-0.03 |
-0.06 |
1 |
||||
|
C18:0 |
-0.36 |
-0.60 |
0.751* |
1 |
|||
|
C18:1 |
0.644* |
0.499 |
-0.44 |
-0.41 |
1 |
||
|
C18:2 |
-0.59 |
-0.71* |
-0.29 |
0.20 |
0.148 |
1 |
|
|
C18:3 n-3 |
0.139 |
0.462 |
-0.54 |
-0.86* |
-0.10 |
-0.43 |
1 |
* Statistically significant correlation (p≤0.05)
Another interesting fact is that C18:0 FA had significant (p≤0.05) negative effect (r=-0.86) on the content of essential ALA (see Table 2). It means that with the increase of C18:0 FA content, content of ALA generally decreased, which was especially obvious in cultivar 1, as already mentioned. Significant negative correlation was found also between C18:2 and C16:0 (r=-0.71), while C14:0 and C16:0 were in significant positive correlation (r=0.883). The FA composition of seed oils varies widely among different species. Usually, the occurrence of some specific FAs is characteristic for particular plant families (Aitzetmüller, 1996; Özcan et al., 2010). The most dominant FA in all examined linseed cultivars was ALA, with mean value of 53.23% (see Table 3). The highest content of this FA was recorded in cultivar number 5 (61.06%). Cultivar 1 significantly differed (p≤0.05) from all other samples by being lower in the content of ALA (42.97%). It is noticeable that this cultivar contained significantly (p≤0.05) higher content of C18:0 FA, which was present in the amount of 19.43%. By comparing the obtained results with those presented in paper of Bayrak et al. (2010), the difference in FA compositions is evident. In the aforementioned paper, authors did not detect C14:0 and C16:1 FAs, while detecting C20:0 and C20:1. Bean and Leeson (2002) reported results more similar to ours, except for absence of C14:0 FA. Specific FA composition of examined Serbian cultivars might be used as one of the benchmarks for authentication of linseed origin.
As shown in the paper of Bean and Leeson (2002), mean PUFA content of 23 examined linseed samples was 71.55%, which is in accordance with our results. However, El-Beltagi et al. (2007) reported lower values-for PUFA content (66.5%) in five linseed cultivars from Egypt. FA composition of linseed oil especially composition of unsaturated FAs and their content highly depends on climatic conditions. Early and late frosts, as well as high temperatures and drought damage kernels, is causing higher content of palmitic (C16:0), linoleic (C18:2) and ALA than it is in whole and undamaged kernels (Sediqi, 2012; Hall et al., 2006). Cool climatic conditions characteristic for northern regions increase the contents of linoleic acid and ALA (Nykter and Kymäläinen, 2006). High humidity conditions extend accumulation period of unsaturated fatty acids (Laza and Pop, 2012).
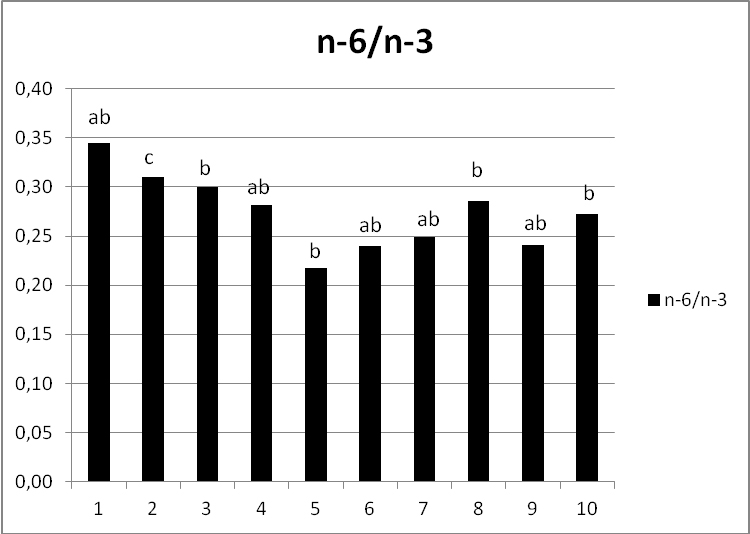
N-6/n-3 ratio in the linseed grains ranged from 0.22 (cultivar 5) to 0.35 (cultivar 1), as presented in Figure 1. Western diets are deficient in n-3 FAs, while having extortionate amounts of n-6 FAs in comparison with the diet on which humans evolved. Recommended values of n‑6/n-3 ratio should be at least less than 4/1 in the secondary prevention of cardiovascular disease, and a ratio of 2-3/1 suppressed inflammation in patients with rheumatoid arthritis (Simopoulos, 2004; Scollan et al., 2006). FA composition of all investigated cultivars can be considered as nutritionally desirable. Although there were significant differences in n‑6/n-3 ratio between the cultivars, all values were far below 2-3/1.
The significance of lipid soluble antioxidants is crucial in protection of lipid-rich food from fast oxidative deterioration. Since linseed is an oilseed, ACL was chosen as an appropriate criterion for determination of antioxidant capacity in linseed kernels, and the results are presented in Table 3.
The highest ACL was recorded in cultivar 10 (342.66 μmol trolox/kg d.m.), and the lowest in cultivar 4 (136.44 μmol trolox/kg d.m.). The ACL values varied over a wide range and significant differences (p≤0.05) were observed among the cultivars (see Table 3).
a-c Different superscripts indicate significant differences (p≤0.05)
|
Linseed cultivar |
ACL (μmol trolox/kg d.m.) |
SD |
CV |
|
1 |
260.14bcd |
16.52 |
6.35 |
|
2 |
273.32cd |
9.23 |
3.38 |
|
3 |
273.55cd |
6.33 |
2.31 |
|
4 |
136.44a |
6.38 |
5.00 |
|
5 |
259.96bcd |
8.64 |
3.32 |
|
6 |
199.26ab |
9.59 |
4.81 |
|
7 |
266.28cd |
5.22 |
1.96 |
|
8 |
250.68bc |
4.50 |
1.80 |
|
9 |
227.65bc |
4.76 |
2.09 |
|
10 |
342.66efg |
10.45 |
3.05 |
|
Mean |
248.99 |
The values are presented as mean, n=3
a-g Different superscripts within the same column indicate significant differences (p≤0.05), SD – standard deviation. CV – coefficient of variation
The cultivars with higher ACL value had stronger protection against oxidation of PUFA. However, statistically significant correlation between ACL and PUFA, or ALA content in the cultivars could not be found. Correlation coefficient between ACL and PUFA content was r=0.04, with p=0.84, while r=0.006 between ACL and ALA, with p=0.99.
The highest PUFA content was recorded in cultivar 5 (Table 3). Nevertheless, ACL value for this cultivar was 259.96 μmol trolox/kg d.m. (Table 3), which was close to the mean ACL value (248.99 μmol trolox/kg d.m.). Cultivar 10 had the highest ACL value, as already mentioned, but this cultivar had PUFA content of 67.36% (mean was 67.66%) and ALA content of 52.92% (mean was 53.62%). It can be concluded that ACL did not depend on the FA composition of linseed, but it might depend on characteristics of a specific cultivar.
Conclusions
Our results showed that the examined Serbian linseed cultivars contained FA C14:0, which is not frequently present in the FA composition of linseed. Therefore, it might be a specificity of linseed oil originated from this specific geographical area. FA C18:0 and ALA were in significant negative correlation (r=-0.86), meaning that with an increase in C18:0 content, ALA content decreased, and vice versa. The highest ACL was recorded in cultivar 10 (342.66 μmol trolox/kg d.m.), and the lowest in cultivar 4 (136.44 μmol trolox/kg d.m.). However, any significant correlation between ACL and content of any FA was not found, which might lead to conclusion that ACL is dependent on linseed cultivar, rather than FA composition.
ACKNOWLEDGEMENTS
The authors are thankful to the Serbian Ministry of Education, Science and Technological Development for financial support - Project No. III 46012. Presented results are also a part of the Project of bilateral cooperation between the Republic of Slovenia and the Republic of Serbia “Influence of thermal treatments on antioxidative capacity, oxidative stability and nutritive value of animal feed“.