Influence of thymol and carvacrol оn initial cell attachment and biofilm of Candida albicans
DOI:
UDK:
JOURNAL No:
Volume 42, Issue 1
PAGES
23-30
KEYWORDS
Candida albicans, biofilm, carvacrol, thymol
Ivana S. Čabarkapa, Marija M. Škrinjar, Jovanka D. Lević, Nevena T. Blagojev, Bojana M. Kokić, Dragana V. Plavšić, Ljiljana Đ. Suvajdžić
1University of Novi Sad, Institute of Food Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia
2University of Novi Sad, Faculty of Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia
3University of Novi Sad, Faculty of Medicine, Department of Pharmacy, Hajduk Veljkova 3, 21000 Novi Sad, Serbia
2University of Novi Sad, Faculty of Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia
3University of Novi Sad, Faculty of Medicine, Department of Pharmacy, Hajduk Veljkova 3, 21000 Novi Sad, Serbia
ABSTRACT
Microorganisms within a biofilm have a distinct phenotype from planktonic cells and generally show higher tolerance to antimicrobial agents. Due to these properties of biofilm-associated cells there is a great interest for finding substances, which inhibit specific processes in the initial phase of biofilm formation and therefore prevent the formation of mature biofilms. Hence, the purpose of this study was to examine the influence of thymol and carvacrol on the planktonic population, initial adhesion and preformed Candida albicans biofilms, in vitro.
Results for antifungal activity of essential oil components (EOC’s) against planctonic population of C. albicans were identical for both tested EOC’s (MIC/MFC=0.156/0.3125 μL/mL). In order to understand the anti-biofilm action of EOC’s, their effect was tested on both the initial cell attachment by planktonic cells as well as on preformed biofilms. Obtained results indicated that the effect of EOC’s on initial cell attachment was dose dependent manner, although even at 1×MIC biomass attachment was reduced by 61.3% for thymol and 58.9% for carvacrol. Using 2×MIC, biomass attachment was reduced for 81.7% for thymol and 80.9% for carvacrol. When the same EOC’s were tested against a preformed biofilm, their inhibitory effect was reduced greatly.
Results for antifungal activity of essential oil components (EOC’s) against planctonic population of C. albicans were identical for both tested EOC’s (MIC/MFC=0.156/0.3125 μL/mL). In order to understand the anti-biofilm action of EOC’s, their effect was tested on both the initial cell attachment by planktonic cells as well as on preformed biofilms. Obtained results indicated that the effect of EOC’s on initial cell attachment was dose dependent manner, although even at 1×MIC biomass attachment was reduced by 61.3% for thymol and 58.9% for carvacrol. Using 2×MIC, biomass attachment was reduced for 81.7% for thymol and 80.9% for carvacrol. When the same EOC’s were tested against a preformed biofilm, their inhibitory effect was reduced greatly.
INTRODUCTION
Candida is the most frequently isolated fungal pathogen in humans causing a variety of difficulties ranging from superficial mucosal infections to systemic mycoses (Pathak et al., 2012). One of the major factors contributing to the virulence of Candida is its ability to adapt to various habitats for growth and formation of surface-attached microbial communities known as biofilms.Biofilms are defined as microbial communities encased in a matrix of extracellular polymeric substance (EPS), which display phenotypic features that differ from their planktonic or free floating cells (Ramage et al., 2005; Cabarkapa et al., 2013). C. albicans biofilms are comprised primarily of yeast-form and hyphal cells, both of which are required for biofilm formation (Finkel and Mitchell, 2011).
Biofilms result from a natural tendency of microbes to attach to biotic or abiotic surfaces, which can vary from mineral surfaces and tissues to synthetic polymers and medical devices, and to further grow on these substrates (Tournu and Van Dijck, 2012). Due to this ability, they constitute a permanent source of contamination, and they can disturb the proper usage of the material onto which they develop and can represent a continuous source of the infection.
Nutrients, quorum-sensing molecules, and surface contact are contributory factors. Fungal biofilm development includes arriving at an appropriate substratum, formation of conditioning layer, adhesion, aggregation, extracellular matrix (ECM) production, biofilm maturation, and dispersion. ECM accumulates as the biofilm matures, and seems to contribute to cohesion. Candida biofilms are significantly less susceptible to commonly used antifungals, moreover, their resistance to antifungals increases with maturation of the biofilm (Dalleau et al., 2008). This makes biofilm-associated C. albicans infections more difficult to treat and has led to an extensive research for new and effective treatments against biofilm associated organisms. Numerous studies have been reported on the antimicrobial potential of plant extracts and essential oils against planktonic bacteria and fungi (Burt and Reinders, 2003; Burt, 2004; Čabarkapa et al., 2012). It has been observed that plant-derived products such as essential oils and their pure components can influence microbial biofilm production (Khan and Ahmad, 2012; Jadhav et al., 2013).
However, less attention has been given to the biofilms which are more resistant to disinfection and therapeutic intervention including antibiotics. Therefore, the purpose of this study was to investigate inhibitory effect of common essential oil components (EOC’s) carvacrol and thymol on the planktonic and biofilm population of C. albicans, in vitro.
MATERIAL AND METHODS
Preparation of fungal suspension
C. albicans ATCC 10231 were cultured on Sabouraud dextrose broth (SDB, LabM) at 37 °C for 48 h. The fungal inoculates were prepared using 48 h old cultures and suspensions were adjusted to 0.5 McFarland standard turbidity.Antimicrobial activity assessment
The antimicrobial activity of EOC’s (carvacrol and thymol) was evaluated using laboratory control strain, Candida albicans ATCC /10231/ obtained from the American Type Culture Collection.Resazurin powder preparation
A stock solution of the resazurin sodium salt (7-Hydroxy-3H-phenoxazin-3-one 10-oxide, Himedia) powder was prepared in sterile distilled water, concentration 0.01%. It was filter-sterilized and kept at 4 °C.Essential oils components
Two pure components of essential oil, phenol monoterpens (carvacrol and thymol), were obtained from Sigma–Aldrich. The purity of carvacrol and thymol was above 98.0% and 99.0%, respectively.Broth microdilution assay
Broth microdilution method was used to determine the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) according to the National Committee for Clinical Laboratory Standards (NCCLS, 2002), with some modifications. The fungal inoculates were prepared using overnight cultures and suspensions were adjusted to 0.5 McFarland standard turbidity.All tests were performed in Sabouraud maltose broth (SMB, LabM). Propylene glycol. (2 - (2 - hydroxypropoxy)-1-propanol) was used to dissolve the EOC’s and then diluted to the concentration (100 - 0.39 μL/mL). Twenty microliters aliquots of the EOC’s were added to 96-well microtitre plates, in geometric dilutions, ranging from 100 to 0.39 µL/mL. Afterwards, aliquots of 160 µL of SMB, were added into each well. As the final step, 20 µL of 2x106 CFU/mL, colony-forming unit (CFU) (according to 0.5 McFarland turbidity standards) of standardized fungal suspensions was inoculated into each microplate. The test was performed in a total volume of 200 µL with final EOC’s concentrations of 10-0.039 µL/mL. Plates were incubated at 25 °C for 24 hours. The same tests were performed simultaneously for growth control (SMB + test organism) and sterility control (SMB + test oil).
After 24 hours of incubation, 20 µL of the resazurin solution was added to each well and the plate was re-incubated overnight. A change of color from blue (oxidized) to pink (reduced) indicated the growth of fungi. The MIC was defined as the lowest concentration of each drug that prevented this change in color.
Referring to the results of the MIC assay, the wells showing complete absence of growth were identified and 100 µL solutions from each well was transferred to Sabouraud maltose agar plates (SMA, Torlak) and incubated at 25 °C for 48 hours. The MFC was defined as the lowest concentration of the EOC’s at which 99.9% of the inoculated microorganisms were killed.
Inhibition of initial cell attachment
The effect of EOC’s on biofilm formation was evaluated as described by Jadhav et al. (2013). Solutions of EOC’s (equivalent to 1×MIC and 2×MIC) were prepared. Twenty microliters of each solution were added to individual wells of a sterile flat-bottomed 96-well polystyrene microtitre plates (Greiner Bio-One). Afterwards, aliquots of 160 µL of SMB were added into each well. As the final step, 20 µL of 2x106 CFU/mL (according to 0.5 McFarland turbidity standards) of standardized fungal suspensions were inoculated into each well to yield a final volume of 200 µL in each well. The cultures were added into the wells in quadruplicate.Control well contained all components except the inoculums (180µL SMB + 20 µLof specific concentration of EOC diluted in propylene glycol).
Positive control well contained 160 µL SMB + 20 µL inoculums (in the same broth) + 20 µL of pure propylene glycol; this control is to reveal potential effect of solvent propylene glycol on Candida growth.
Sterility control well contained 180 µL SMB + 20 µL pure propylene glycol; this control is to reveal possible contamination of solvents.
The plates were sealed and incubated for 24 hours at 25 °C under sterile conditions to allow cell attachment. Biofilm formation was assessed using the crystal violet (CV) assay described by Agarwal et al. (2011).
Inhibition of preformed biofilm
The effect of essential oil components on biofilm growth and development was evaluated as described by Jadhav et al. (2013), with some modifications. Biofilms were allowed to be formed for 6 hours prior to addition of EOC’s. Biofilm formation was achieved by transferring 160 µL of SMB into each microplate, followed by addition of 20 µL of fungal culture (prepared as described above) into the wells of sterile flat-bottomed 96-well polystyrene microtitre plates in quadruplicates.The scheme of control samples was the same as described in previous section.
The microtitre plates were covered and incubated for 6 hours at 25 °C to allow cell attachment and biofilm formation. Following incubation, 20 µL of each stock solution of EOC’s was added to each well to yield a final volume of 200 µL. After the treatment of preformed biofilms with EOC’s, the plates were incubated for 5 hours and 24 hours. Following incubation, the biofilms were assessed for biomass attachment using the CV assay.
Biofilm biomass assay (modified crystal violet assay)
Indirect assessment of cell attachment for Candida albicans was evaluated using the modified crystal violet (CV) assay described by Agarwal et al. (2011). Following the 24 hours incubation (Section Inhibition of initial cell attachment) and the 5 hours and 24 hours incubation (Section Inhibition of preformed biofilm), culture medium from each well was gently removed and the plates were washed three times with 250 µL sterile distilled water to wash away any loosely attached cells. The plates were air dried for 45 minutes. The cells in the biofilm were then stained with 250 μL 0.3% crystal violet and incubated at room temperature for 15 minutes. The stain was removed by exhaustive washing with distilled water.
The plates were then allowed to dry. In order to quantify adhered cells, 250 μL of decolouring solution (ethanol/acetone, 80:20%) was added to each well for 15 minutes. The absorption of the eluted stain was measured at 595 nm using a microplate reader (ChemWel, Awareness Technology). The median absorbance (OD595 nm) was used for determining the percentage inhibition of biomass formation for each concentration of the oil according to the following equation:

Statistical analyses
Statistical analyses were performed by Statistica 12 (StatSoft Inc., Tulsa, Oklahoma). Due to of size of the sample (n<30), the data from the assays were compared using the nonparametric Mann-Whitney test. Results was considered to be statistically significant at p<0.05.
RESULTS AND DISCUSSION
Ability of Candida spp. to form biofilms on abiotic and biotic surfaces has been demonstrated in many researches (Watamoto et al., 2009; Boucherit-Atmani et al., 2011; Khan and Ahmad, 2012; Pathak et al., 2012). Resazurin is an oxidation–reduction indicator used for the evaluation of cell growth, particularly in various cytotoxicity assays. It is a blue non-fluorescent and non-toxic dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. Resorufin is further reduced to hydroresorufin (uncoloured and nonfluorescent). A resazurin reduction test has also been used for decades to demonstrate bacterial and yeast contamination of milk (Sarker et al., 2007).Results for antifungal activity of EOC’s obtained using broth microdilution method with resazurin against C. albicans were identical for both tested EOC’s (MIC/MFC = 0.156/0.3125 μL/mL). These results are in agreement with the findings of Ahmad et al. (2011). They found the MIC values of thymol ranging from 0.1 to 0.15 μL/mL against Candida species. In the same study, MIC values of carvacrol were slightly lower and ranging from 0.075 to 0.1 μL/mL. In the research of Braga et al. (2008), MIC of thymol for two Candida species was 0.125 μL/mL (Braga et al., 2008). Ahmad et al. (2011) hypothesized that EOC’s penetrate into the cell and target the ergosterol biosynthesis pathway, thus, impairing its biosynthesis. Simultaneously, they react with the membrane itself with their reactive hydroxyl moiety, and the extensive lesion on the membrane is a combined effect of the two events. In order to understand the anti-biofilm action of EOC’s, their effect was tested on both the initial cell attachment by planktonic cells as well as on preformed (6 hours) biofilms. For clarity, results were expressed as inhibition percentages of biofilm development (Figure 1, 2 and 3). The CV assay indicated that the effect of EOC’s on initial cell attachment was dose dependent manner, although even at 1× MIC biomass attachment was reduced by 61.3% for thymol and 58.9% for carvacrol. However, complete inhibition of cell attachment was not achieved despite using 2 × MIC of EOC’s. Using 2 × MIC, biomass attachments were reduced for 81.7% for thymol and 80.9% for carvacrol (Figure 1). A similar mode of action of EOC’s against initial adhesion of C. albicans was observed in other study (Khan and Ahmad, 2012).
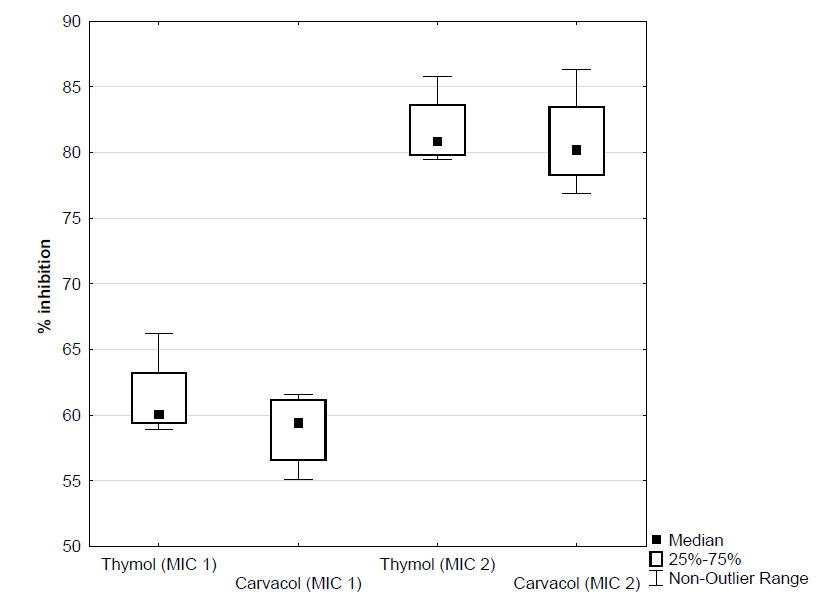 Figure 1. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on initial cell attachment
Figure 1. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on initial cell attachment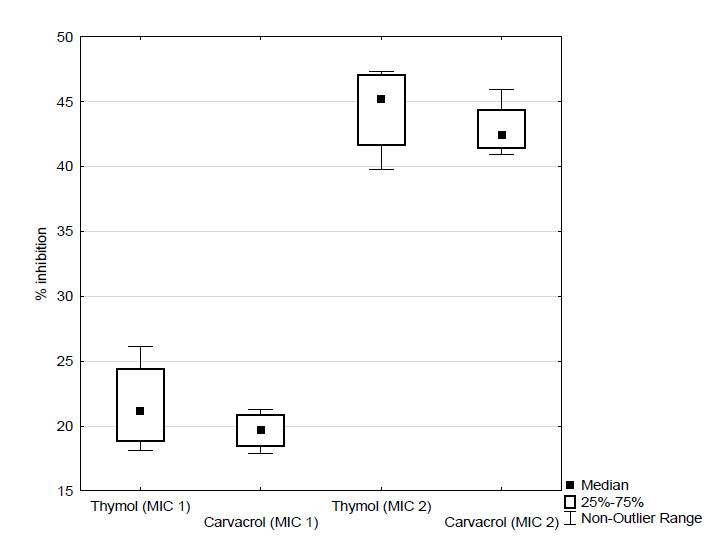 Figure 2. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on 6 hours preformed biofilms of C. albicans incubated with the EOC’s for 5 hours
Figure 2. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on 6 hours preformed biofilms of C. albicans incubated with the EOC’s for 5 hours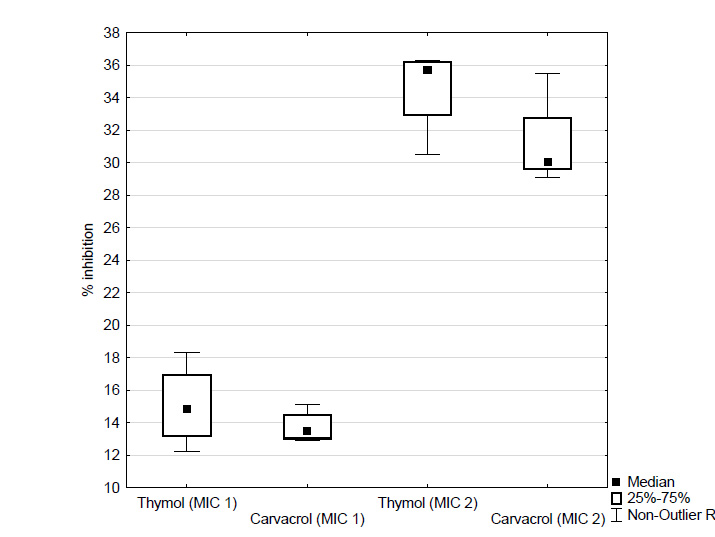 Figure 3. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on 6 hours preformed biofilms of C. albicans incubated with the EOC’s for 24 hours
Figure 3. Effect of different concentrations of thymol and carvacrol (expressed as percentage inhibition of biofilm formation) on 6 hours preformed biofilms of C. albicans incubated with the EOC’s for 24 hoursAnother factor which may contribute to this increased resistance is that the majority of antimicrobial compounds are more effective against actively growing cells. The cells in a biofilm have a poor growth rate due to lack of nutrients and oxygen, which may reduce the antimicrobial effects of compounds against them (Sandasi et al., 2010). Due to excellent inhibitory effect of EOC’s on initial cell attachment, the use of these components with a view to the preventive inhibition of biofilm formation is a promising approach.
CONCLUSIONS
Present investigation demonstrated that EOC’s are effective not only on planktonic cells but also on biofilms of Candida albicans that are resistant to many antifungal drugs. Thus, it was concluded: a) overall, EOC’s were found to be more effective in inhibiting initial cell attachment compared to preformed biofilms; b) thymol was more active than the carvacrol; c) these bioactive components would allow inclusion of these compounds in novel pharmaceutical products, disinfectant and sanitizer formulations.АCKNOWLEDGEMENTS
This paper is a result of research within the projects III 46009 and III 46012 financed by the Ministry of Education, Science and Technological Development, Republic of Serbia. Part of this work was supported by the COST ACTION FA1202 BacFoodNet.


