Introduction
The occurrence of t FAs in foods has been heavily discussed in the last few years not only in professional journals but also in the daily press because of the relevance of these compounds for human health. Numerous adverse effects have been reported. Most attention has been drawn to the enhancement of low density lipoprotein cholesterol and the decrease of high density lipoprotein cholesterol levels in blood and in connection with this to the increased risk for cardiovascular diseases (Hunter et al., 2010; Micha and Mozaffarian, 2008; Wallace and Mozaffarian, 2008). Also elevation of insulin resistance with resulting diabetes and higher weight gain, increased risk of colorectal cancer and a disadvantageous influence on haemorheological parameters may be observed (Mozaffarian et al., 2008; Kato et al., 2010; Tai et al., 2010).
Ones of the most relevant sources of t FAs in the past were partially hydrogenated vegetable oils. But importance has also to be assigned to the formation of t FAs in foods as a result of heat processing. Roasting in particular appears to be a critical process for the formation of t FAs. Significant levels of between 0.6 and 0.9 g/100 g were found in roasted nuts (Yaacoub et al., 2008). Higher quantities of t Fwere observed in consequence of the deodorization process of rape seed oil. In one example of this process 250 °C was applied for 5 to 6 hours with the result that more than 5% of the total fatty acids were isomerized to t F (Lamblet et al., 2003). There are also some hints about the important role of matrix components in literature. In solutions and micelles of soybean lecithin thiyl radicals could be identified as catalysts for the cis trans isomerisation of unsaturated fatty acids. The reaction was accelerated by oxygen and inhibited by conjugated dienes like all-trans-retinol (Ferreri et al., 2001). These results were confirmed by in vivo trials with rats, which were able to demonstrate the differentiated inclination of tissues for t-FA formation in living organisms due to free radicals, and the inhibition of this process by all trans-retinol (Zambonin et al., 2006). The importance of thiyl radicals for the formation of t-FA in the membranes of ery-throcytes and the protective effect of antioxidants such as alpha tocopherol under the influence of gamma irradiation could also be demonstrated (Ferreri et al., 2002). Other studies have found trans-arachidonic acids in human blood plasma in mean concentrations of 20.2 ng/ml. The occurrence of t-FA is attributed by the authors to the catalytic activity of nitrogen dioxide radicals (Zghibeh et al., 2004). This is obviously in contradiction to the generally accepted opinion that the contribution of nitrogen dioxide radicals to the isomerisation of UFA is very low (Chatgilialoglu and Ferreri, 2005; Chatgilialoglu et al., 2006). The mechanism of isomerisation induced by thiols or by the corresponding thiyl radicals could be elucidated in more detail by experiments with homogenous solutions and suspensions of unilamellar vesicles of different lecitins. In solutions the isomerisation was found to be randomly distributed in the four double bonds of arachidonic acid while in membrane bilayers there was a preference for the isomerisation of the double bonds situated nearest to the polar region of the molecule (Ferreri et al., 2004). Further on there is some influence of the polarity of the catalysing thiyl radical. It could be demonstrated that cis–trans iso merisation is enhanced by increasing the hydrophobicity of thiols (Sprinz et al., 2001). On top of this the influence of the situation of the double bond and the hydrophobicity of the catalyst may be understood as a pointer to the role of catalysts and their ability to reach the double bonds in the molecule. There is a lot of evidence about the formation of t-FA s in biological systems and the role of thiols or the ensuing thiyls as catalysts, but most studies concern model systems, blood, animal or human cells. Until now we are not aware of any evidence about formation of t-FA in plant material. The formation of radicals is favoured by the presence of non-polar solvents such as vegetable oils. In natural plant tissue the oils are in contact with a lot of organic compounds capable of forming radicals such as disulfide bonds in protein or glutathione in its oxidised form. By the influence of elevated temperatures the originnation of radicals is enhanced. Thus ideal conditions may be expected for the formation of t-FA by the contact with catalysing radicals. The present investigations are aimed at finding out whether components of plant tissue have any catalytic effects on the formation of t-FA and to quantify these effects.
MATERIALS AND METHODS
Origin of the samples: Seed samples of linseed, rape, soybeans and sunflowers originnating in variety testing trials harvested in 2007. The selected varieties were: Rezital (linseed), Californium (rapeseed), Cardiff (soybeans) and Alexandra (sunflowers). Pumpkin kernels were bought from a storage company. The vegetable oils were unrefined and cold pressed. Soyabean (Vitaquell, Hamburg, Germany) and linseed oil (Neuco, HEIRLER CENOVIS G.m.b.H. Radolfzell, Germany) originated from organic farming. Oil from pumpkin, sunflower and rape seeds was bought from Fandler, Austria. The oils were stored at 4 °C in the dark.
Heating trials: Oils and seeds of all species were exposed to heat at 423 K, 473 K and 523 K for 30, 60, 120 and 180 min in an incubator with mechanical air circulation. At 573 K the samples were exposed for 30 and 60 min. 10 min was added to the exposure times to heat the samples to the defined temperature. From all oils and seeds un heated samples were also reserved for GC analysis. The heating trials were performed as follows: 3g +/ 0.2g of seeds or 1 g of oil were weighed into porcelain crucibles. The crucibles were covered with watch glasses, stored in an incubator with recirculating air (WTB Binder, Tuttlingen, Germany) and tempered to the selected temperature. After exposure to heat the samples were cooled down to room temperature. For the determination of losses of volatile substances oil samples were weighed before and after heating. The seeds were milled in a coffee grinder after cooling.
Esterification of samples: The method is based on the direct saponification of FAs in the oil or in the ground sample with methanolic NaOH and esterification with boron triflouride (Rückemann, 1978). 1g +/ 0.1g of sample or 400 µl of oil were suspended or dissolved in 10 ml of 0,5 M methanolic NaOH (methanol p.a. Merck, Darmstadt, Germany, NaOH Fluka, Buchs, Switzerland) and boiled. After 8 min 7 ml of methanolic solution of boron trifluoride (10% w/w Fluka) were added to the boiling liquid. After a further 8 min 10 ml n hexane (Optigrade, Merck) were added and the FA methyl esters were extracted by boiling for one further minute. The flasks were cooled down in cold water and filled with saturated NaCl (p.a. Merck) solution until the n hexane layer was up to the bottle neck. 1 ml was taken from the n-hexane and diluted to 5 ml. To remove traces of water a small quantity of water free Na2SO4 (p. a. Merck) was added. The resulting n hexane layer was ready for GC analysis.
GC analysis: FAMEs were separated and quantified on a GC-system HP 6890 equipped with a bis(cyanopropyl) -siloxane column 100 m x 0.25mm ID, 0.2µm film thickness, HP-88 (Agilent, Model Nr.112-88A7) (Frank and Pat, 2005). The parameters for the GC analysis were as follows: Injector temperature 250 °C; Injection volume 0.5µl; Split ratio 10:1; flow velocity of carrier gas 18 cm/sec at 34 psi. A temperature program with initial temperature 150 °C with equilibration time of 10 min followed by a gradient of 0.75 °C/min up to 205 °C continued by a temperature gradient of 2 °C/min up to a final temperature of 240 °C and final time of 10 min was used.
For detection of FA-methylesters by FID nitrogen was used as carrier and makeup gas. The detector temperature was adjusted to 250 °C. The following gas flows were applied: Hydrogen 40 ml/min; synthetic air 450 ml/min; makeup-gas (nitrogen) 30 ml/min.
Mass spectroscopy was used for the identification of peaks as FA. For this purpose the same equipment and GC procedure was applied, except the use of helium as carrier gas. A Trace GC-DSQ II (Thermo-Scientific-Fisher, Waltham, Massachusetts) single quadrupole mass spectrometer equipped with the software Xcalibur Rev. 2.0.7 was used as detector instead of the FID. The ionisation mode was electron impact, the acquisition mode was scan 20 – 550 amu. Ionisation energy was 70 eV and ion source temperature was at 230 °C, the transferline temperature was at 280 °C. The results were verified by GC-MS in the case of linseed and linseed oil, because these materials contain all three UFAs usually present in plant material in reasonable quantities.
The identification of peaks was made by comparing retention times with those of standard isomer mixtures available on the market. For identification of peaks the following methyl ester standards were used: F.A.M.E. mix rapeseed oil (SUPELCO/-SIGMA-ALDRICH, St Louis, Missouri,), trans-9-elaidic methyl ester (SUPELCO/SIGMA-ALDRICH), methyloleate (Fluka, Buchs, Switzerland), linoleic acid methyl ester isomer mix (SUPELCO/SIGMA-ALDRICH), linolenic acid methyl ester isomer mix (SUPELCO/SIGMA-ALDRICH).
According to the method of calibration the results were obtained for each FAME as area % from the whole quantity of FAMEs present in the sample.
Evaluation of results: For the mathematical exclusion of the influence of losses of PUFAs on the formation of t-FA the amount of trans-isomers had to be set in relation to the amount of the all-cis form of the UFA from which they are derived. So the sum of all isomers of the fatty acid at the time of measurement was set at 100% and the sum of all appropriate trans-isomers was expressed as a percentage of the whole quantity of the respective FA. This procedure was done separately for each repetition of the trials, so that mean values and standard deviations could be calculated. All trials were done in duplicate.
The performance of heating trials at different temperatures allows also the calculation of activation energies for the cistrans isomerisation. Therefore the rate constants k1 for T1 (573 K) and k2 for T2 (523 K) were calculated as an average of the increase of TIs during all periods from 30 min to 180 min of the heating trials. The activation energy Ea was found according to the following procedure: the two temperatures and their corresponding rate constants were substituted into the logarithmised Arrhenius Equation (1 and 2).
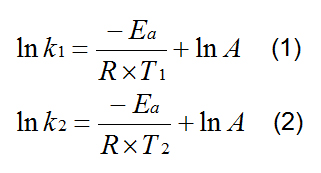
A = pre-exponential factor, R = 8,314 [J mol-1 K-1]. By rearrangement and solution of Ea equation 3 is found by subtracting equation 2 from equation 1.
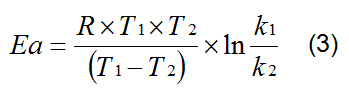
Standard deviations and significances of differences by Students t-test were calculated by Microsoft-EXCEL 2002.
RESULTS AND DISCUSSION
Limit of detection and quality of chromatograms: In chromatograms recorded by FID, which were used for quantifying the fatty acids, all peaks considered in this study reached an S/N of more than 20 because of a very stable base line. This means, that also the smallest peaks were quantifiable. GC/MS, which was not used for quantification of peaks, but only for identification of substances, showed according to the nature of the method a much higher noise. An S/N of 3 was assumed as lower limit of detection. The smallest peaks of FA mentioned in this paper – for example trans-9, cis-12-linoleic acid ME in linseedoil after 120 min at 523 K (peak 7 in Fig. 1A) - reached an S/N of more than 3. Fatty acid composition of raw materials: The oil and seed samples represent the typical FA profile of the crops selected for this study (Tab.1). The results show higher standard deviations between double determinations in pumpkin and sunflower seeds caused by the kernel size of these two crops. Besides the five FAs mentioned in the table some minor components were also found. They mainly correspond to arachic, eicosenoic and behenic acid. An undefined compound appearing immediately after OLA in the chromatograms was found in fresh and heat-treated material. It was identified by GC/MS as a FA. According to published chromatograms this peak might correspond to 11-cis OLA (Tsuzuki et al., 2008).
Losses of PUFAs during heating trials: Only with reference to the subject of this study, the degree of isomerisation of UFAs in oils and seeds, losses are of interest. There is the question, whether there is a difference in the tendency to oxidation between cis- and trans-fatty acids, which might contribute to an apparent enrichment of one group of the compounds. A study of baking trials does not find any distinction between the two kinds of isomers with reference to oxidation (Zbikowska and Kowalska, 2007). On the other hand OLA absorbed under the influence of photosensitation by methylene blue double the quantity of oxygen as ELA (Lee et al., 2010). The results of the baking trials seem to be more representative of the behaviour of UFAs in practice, because they included all three kinds of UFAs in vegetables and most notably the oxidation was not based on the action of a special catalyst such as methylene blue. Based on the enhanced damage of PUFAs - mainly by oxidation - in oils, as could be estimated by the ratio SFA/PUFA, also an enrichment of t-FAs in oils would have to be expected, if cis-isomers would be oxidised more rapidly than trans-isomers. This was evidently not the case. Furthermore the oxidation of trans-fatty acids seems easier by thermodynamic means, because the trans-configuration of the double bonds is more stable than the cis-formation and the placement of electrons in the p-orbit of the double bond on a low energetic level is responsible for the reactivity of the protons on the methylene groups between the double bonds. It may be concluded, that there are a lot of practical and theoretical reasons, that the enrichment of trans-isomers is not a side effect of enhanced damage of cis-isomers. Formation of trans-fatty acids in oils and seeds: The enhanced formation of t FAs in seeds is demonstrated by comparison of the two GC/MS chromatograms in Fig. 1 A and B representing separations of linseed-oil-ME and linseed-ME respectively heated for 120 min at 523 K. The distribution of heat-originated t FAs within the different kinds of isomers seems not to be accidental but to follow a distinct pattern.
Tab. 1. Fatty acid composition of oils and seeds used for heating trials *
|
|
Linseed
|
Pumpkin
|
Rapeseed
|
Soybean
|
Sunflower
|
|
FA-ME
|
area%
|
SD
|
area%
|
SD
|
area%
|
SD
|
area%
|
SD
|
area%
|
SD
|
|
Oil
|
|
PAM
|
5.3
|
0.1
|
12.1
|
0.1
|
4.5
|
0.1
|
11.0
|
0.1
|
6.4
|
0.2
|
|
STA
|
4.1
|
0.0
|
5.5
|
0.1
|
1.8
|
0.0
|
4.7
|
0.0
|
3.9
|
0.1
|
|
OLA
|
19.7
|
0.3
|
28.8
|
0.2
|
63.0
|
0.7
|
21.4
|
0.4
|
19.7
|
0.3
|
|
LNA
|
18.3
|
0.2
|
53.0
|
0.3
|
18.7
|
0.3
|
53.1
|
0.0
|
69.2
|
0.7
|
|
ALA
|
52.3
|
0.2
|
|
|
8.2
|
0.1
|
8.1
|
0.1
|
|
|
|
Seeds
|
|
PAM
|
6.4
|
0.0
|
11.7
|
0.1
|
4.9
|
0.1
|
11.3
|
0.1
|
6.9
|
0.3
|
|
STA
|
4.4
|
0.0
|
6.1
|
0.2
|
1.8
|
0.1
|
4.8
|
0.0
|
3.6
|
0.3
|
|
OLA
|
21.3
|
0.1
|
35.9
|
1.8
|
58.1
|
0.2
|
21.2
|
0.4
|
28.4
|
2.2
|
|
LNA
|
15.5
|
0.1
|
44.1
|
1.1
|
20.8
|
0.0
|
53.2
|
0.0
|
59.4
|
1.6
|
|
ALA
|
51.0
|
0.1
|
|
|
8.3
|
0.1
|
8.1
|
0.3
|
|
|
* All trials were done in duplicate
Most prone to isomerisation seem to be the double bonds proximate to the w-end of the carbon chainfollowed by the double bond next to the carboxyl-group, as is demonstrated by the example of linseed and linseed oil heated up to 523 K (Tab.2).
As an explanation for the preferred trans-isomerisation of the double bonds nearest to the end of the carbon chain the easier accessibility of the w- proximate double bond to catalysing agents may be posited. The favoured isomerisation of the double bonds in the 12 position of LNA and 15 position of ALA would be in good accordance with the increase of effectiveness of catalysts with increase of their hydro-phobicity (Sprinz et al., 2009). The double bond next to the polar region is most prone to isomerisation only in lipid bilayers, which might be caused by the proximity to the surface of these structures (Ferreri et al., 2004). Studies concerning model systems of LNA isomerisation by irradiation in micelles have found almost equal amounts of trans double bonds in 9 and in 12 position, which confirms our observation that the w proximate double bond as well as the one nearest to the polar end are the dominant compounds after heat induced cistrans isomerisation (Mihaljevic et al., 2011). With increasing heating time there is a shift of t LNA with trans 12 position to such trans-isomers with trans-9 position, a fact which is difficult to explain on the basis of existing models. For t ALA only weak separation of some isomers was possible. While it was possible to identify cis-9, cis 12,trans-15 linolenic acid (peak 10 in Fig. 1 A,B) unambiguously, all isomers with two double bonds in transposition had to be merged (peak 9 in Fig. 1 A, B). Also the small peak representing the cis-9,trans-12, cis-15 isomer was merged with the predominant trans-9, cis 12, cis 15 linolenic acid (peak 11 in Fig.1 A,B) to avoid over interpretation because these two peaks were not separated at the base line. The small area corresponding to the ALA-trans-12 isomer indicates the low share of these isomers in the isomer mixture resulting from heat treatment. By far the least share of trans-isomers represented by a base line separated peak was found in ALA with two double bonds in trans-configuration (peak 9 in Fig.1 A).
The exact position of these double bonds also could not be ascertained, because these isomers have nearly all the same retention time, but the sum of these compounds correlates strongly (R2 > 0.99) with the time of heat exposure, which shows that t ALA with two double bonds in trans-configuration accumulates from proceeding isomerisation of t ALA with one double bond.
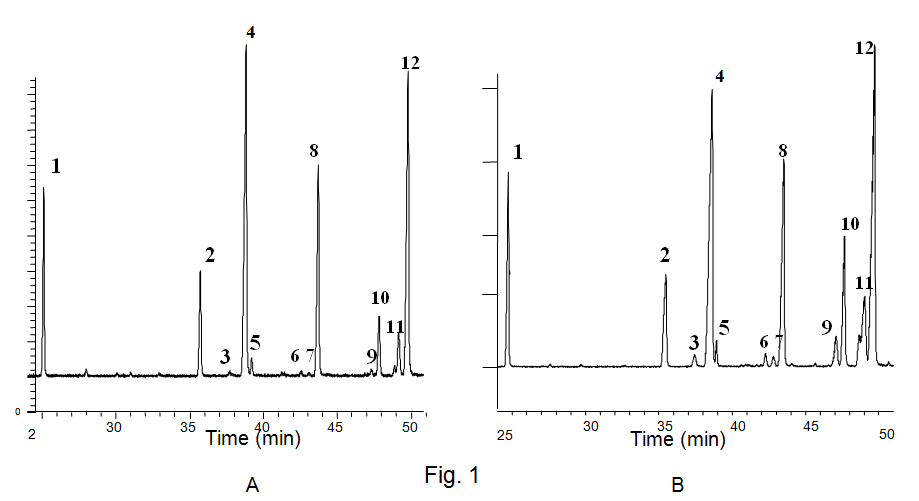 Fig. 1.
Fig. 1. GC/MS-chromatogram of linseed oil-methylesters (A from oil, B from seeds), heated 120 min/523 K. 1 = palmitate, 2 = stearate, 3 = elaidic acid, 4 = oleic acid, 5 = undefined fatty acid, 6 = cis-9, trans-12-linoleicacid, 7 = trans-9,cis-12-linoleic acid, 8 = all-cis-linoleic acid, 9 = 2xtrans, 1xcis-linolenic acid, 10 = cis-9, cis-12, trans-15-linolenic acid, 11 = cis-9, trans-12, cis-15- and trans-9, cis-12, cis-15-linolenic acid, 12 = all-cis-linolenic acid
Tab. 2. Share of different types of trans-isomers on the sum of trans-isomers originating from linoleic and linolenic acid at 523 K in linseed and linseed oil [area %]
|
|
time of heat exposure [min]
|
linoleic-acid trans-isomers
|
linolenic-acid trans-isomers
|
|
cis-9, trans-12
|
trans-9, cis-12
|
2xtrans, 1xcis
|
cis-9, cis-12, trans-15
|
cis-9, trans-12, cis-15 +
trans-9, cis-12, cis-15
|
|
Oil
|
30
|
67
|
33
|
0
|
64
|
36
|
|
60
|
60
|
40
|
2
|
61
|
37
|
|
120
|
55
|
45
|
5
|
50
|
45
|
|
180
|
53
|
47
|
8
|
48
|
44
|
|
mean (oil)
|
59
|
41
|
4
|
56
|
40
|
|
Seeds
|
30
|
67
|
33
|
4
|
63
|
33
|
|
60
|
60
|
40
|
5
|
55
|
40
|
|
120
|
59
|
41
|
9
|
54
|
37
|
|
180
|
56
|
44
|
11
|
50
|
39
|
|
mean (seeds)
|
60
|
40
|
7
|
56
|
37
|
The distribution of different types of trans-isomers in oils and seeds is principally similar. Only the shares of t FAs with two double bonds in trans configuration, are in seeds slightly but significantly higher (P<0.001) than in oils mainly at the costs of the tran -9, cis 12,cis 15 isomer, which may be interpreted as a hint to the enhancement of heat induced isomerisation by some compounds of the matrix. All-trans-isomers were not found at 523 K and only in traces at 573 K. At 423 K there was no formation of t-FA within the maximum time of observation of 180 min, whether in oils nor in seeds. Also at 473 K no origination of t-FA was observed in oils of all crops investigated and in soybeans. Only in linseed oil traces of 0.6 area %, based on the whole quantity of ALA, of cis-9, cis-12, trans-15 ALA after 180 min of heating could be found in one of the double determinations.
Detectable formation of t-FAs at 473 K was observed in the seeds of pumpkin, rapeseed and sunflower. Because of the low level or absence of ALA in these crops only ELA and t-LNA could be identified. The most susceptible crop to formation of t-FAs rapeseed seemed to be, where – similar to sunflowers – preferably OLA was isomerised to ELA.The formation of t-LNA in oils began after 60 min of heat exposure, while in seeds after 30 min a quantifiable amount of LNA was converted to trans-isomers. Also the isomerisation of ALA in oils was lower than in seeds in all cases, but besides the absence of t-ALA in rapeseed oil mentioned above, the difference between oils and seeds was low compared to that observed for ELA and t-LNA. In seeds the highest transition of OLA to ELA was found in rapeseed. The shares of ELA in all other crops followed at a distance with linseed being the lowest one. Also the transition of LNA to t-LNA was twice as high in rapeseed as in all other crops. The rate of isomerisation of ALA seems to be nearly the same in seeds of all crops containing this FA. In linseed and soybeans the difference between t-ALA in oils and seeds is still evident but to a much lesser extent than for isomers of OLA and LNA.
The results of the heating trials at 573 K confirm the results at 523 K (Fig. 3). ELA occurred only in small amounts in oils, while the share of ELA and t-LNA in seeds after 60 min is in the same range or slightly higher as at 523 K after 180 min.
The difference between the shares of t-LNA in oils and seeds was lower at the elevated temperature. Nevertheless the shares of t-LNA on all LNA isomers in oils ranged up to half that observed in seeds, so the big difference between oils and seeds is still evident. Additionally, the relatively small differences in the share of t-ALA between oils and seeds were found.
There is a lot of evidence that different kinds of radicals catalyse the formation of t-FAs. Most studies assign the catalytic effect to thiyl radicals, which might originate from thiols unfolding their potential as antioxidants in seeds changed thereby to thiyl radicals (Ferreri et al., 2002; Ferreri et al., 2004; Sprinz et al., 2001; Mihaljevic et al., 2011).
The presence of thiyl radicals in the matrix of seeds may be considered as a plausible explanation for the enhanced formation of all kinds of t-FAs in comparison to the lower trans-isomer formation in oils where only traces of sulphur compounds are present.
The sulphur metabolism in rapeseed in the course of formation of glucosinolates may be a source of thiyl radicals and be considered as a reason for the higher shares of t-FAs originating in these crop due to heat treatment.
The levels of t-FAs in rapeseed oil are in the same range or lower than in the oils from other crops, which might be explained by the absence of sulphur compounds (Fig. 2 and 3).
The activation energy (Ea) could be calculated only in cases where t-FAs were found at 523 and 573 K (Tab. 3). The lowest Ea together with low pK-values for isomerisation of OLA and LNA were found in rapeseed and rapeseed oil. On the other hand, in linseed oil Ea = 200 KJ mol-1 for the formation of t-LNA which is near to the theoretical value of the p-bond of 265 KJ mol-1and also in seeds the higest value for Ea was found in linseed.
Although the percentages of FA isomerised in linseed seem to be similar to the ones in pumpkins, soybeans and sunflowers (Fig. 2.and 3), relatively high values of Ea together with higher pK-values indicate that there is less catalytic activity. Nearly all activation energies found in this study are lower than the value found in a publication about the isomerisation of OLA in a model system of pure triolein without catalyst, which ranged around 172 KJ mol-1(Christy and Harrington, 2008) and indicates the catalytic activity of some compounds in complex systems as there are oils and especially seeds. E.g. in rapeseed the comparatively low value of Ea confirms that trans-isomerisation is more facilitated by catalytic effects than in other crops.
On the other hand it may be argued that the high amounts of ALA present in linseed and its oils may act as a radical catcher, which might explain that the highest values for Ea for the isomerisation of all kinds of UFA are found in this crop.
The small differences in the formation of t-ALA in oils and seeds of all crops are in good accordance with the small differrences of Ea. In both, oils and seeds, the Ea necessary for isomerisation of ALA was lower than for any other FA.
Additionally the rate of formation of trans-isomers was higher than for ELA and t-LNA, as may be seen on the low pK-values and the high shares of t-ALA formed during heat treatment.
All this seems to indicate that ALA is a very instable compound itself, susceptible not only to oxidation but also to isomerisation and so the acceleration of isomerisation by catalysts is not as effective as for other UFAs.
Tab 3. Estimation of pK-values and activation energy Ea
|
|
|
Elaidic Acid
|
Linoleic trans isomers
|
Linolenic trans isomers
|
|
|
|
pK
(523 K)
|
Ea
[KJ mol-1]
|
pK
(523 K)
|
Ea
[KJ mol-1]
|
pK (523K)
|
Ea [KJ mol-1]
|
|
Oil
|
Linseed
|
|
|
3.53
|
200
|
2.47
|
89
|
|
Pumpkin
|
3.95
|
129
|
3.64
|
169
|
|
|
|
Rapeseed
|
3.59
|
75
|
|
|
|
|
|
Soybean
|
3.53
|
107
|
3.33
|
137
|
2.37
|
77
|
|
Sunflower
|
|
|
3.54
|
156
|
|
|
|
Seed
|
Linseed
|
3.41
|
112
|
2.99
|
120
|
2.38
|
82
|
|
Pumpkin
|
3.32
|
100
|
2.96
|
109
|
|
|
|
Rapeseed
|
2.85
|
69
|
2.70
|
86
|
2.34
|
83
|
|
Soybean
|
3.13
|
94
|
3.00
|
114
|
2.21
|
73
|
|
Sunflower
|
3.28
|
103
|
2.91
|
105
|
|
|
|
Difference between oil and seeds
|
Linseed
|
|
|
0.54
|
80
|
0.08
|
7
|
|
Pumpkin
|
0.63
|
29
|
0.68
|
60
|
|
|
|
Rapeseed
|
0.73
|
6
|
|
|
|
|
|
Soybean
|
0.39
|
13
|
0.34
|
23
|
0.16
|
4
|
|
Sunflower
|
|
|
0.63
|
51
|
|
|
CONCLUSION
The enhancement of t-FA-formation in seeds could be demonstrated to be evident. The estimation of Ea confirms by the differences of this value between oils and seeds that the enhanced formation of t FAs in seeds is based on catalytic effects. Therefore the catalytic agent has to be localized in the matrix of the samples.
A lot of antecedent studies describe the role of thiyl radicals in connection with cis/trans isomerisations of UFAs in model systems and animal tissue. This leads to the conclusion that sulphur compounds in the matrix of the seeds are responsible for the increased formation of t FAs.
For the technological practice it may be concluded that only excessive heat treatment may cause the origination of t-FAs acids. Especially roasting of oil containing seeds has to be designed considering possible t FA formation. Oils are less susceptible to tFA formation, but extended heating to high temperatures, as might occur in the course of refining processes, should be avoided.
ACKNOWLEDGEMENTS
The financial support of Ströck-Brot Ges. m.b.H., Vienna, Austria is gratefully acknowledged.
Download full article PDF
 DOWNLOAD PDF
DOWNLOAD PDF
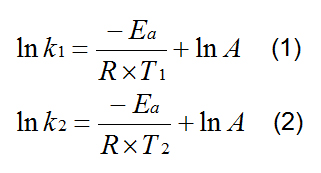
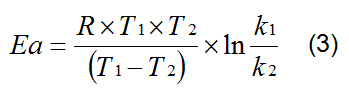
 Fig. 1. GC/MS-chromatogram of linseed oil-methylesters (A from oil, B from seeds), heated 120 min/523 K. 1 = palmitate, 2 = stearate, 3 = elaidic acid, 4 = oleic acid, 5 = undefined fatty acid, 6 = cis-9, trans-12-linoleicacid, 7 = trans-9,cis-12-linoleic acid, 8 = all-cis-linoleic acid, 9 = 2xtrans, 1xcis-linolenic acid, 10 = cis-9, cis-12, trans-15-linolenic acid, 11 = cis-9, trans-12, cis-15- and trans-9, cis-12, cis-15-linolenic acid, 12 = all-cis-linolenic acid
Fig. 1. GC/MS-chromatogram of linseed oil-methylesters (A from oil, B from seeds), heated 120 min/523 K. 1 = palmitate, 2 = stearate, 3 = elaidic acid, 4 = oleic acid, 5 = undefined fatty acid, 6 = cis-9, trans-12-linoleicacid, 7 = trans-9,cis-12-linoleic acid, 8 = all-cis-linoleic acid, 9 = 2xtrans, 1xcis-linolenic acid, 10 = cis-9, cis-12, trans-15-linolenic acid, 11 = cis-9, trans-12, cis-15- and trans-9, cis-12, cis-15-linolenic acid, 12 = all-cis-linolenic acid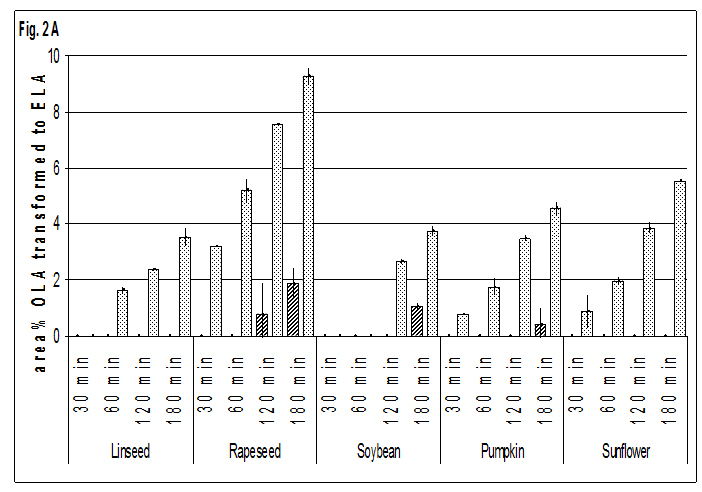
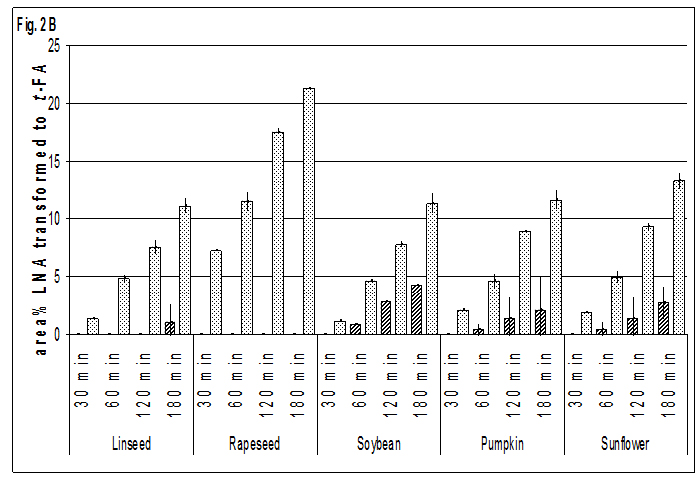
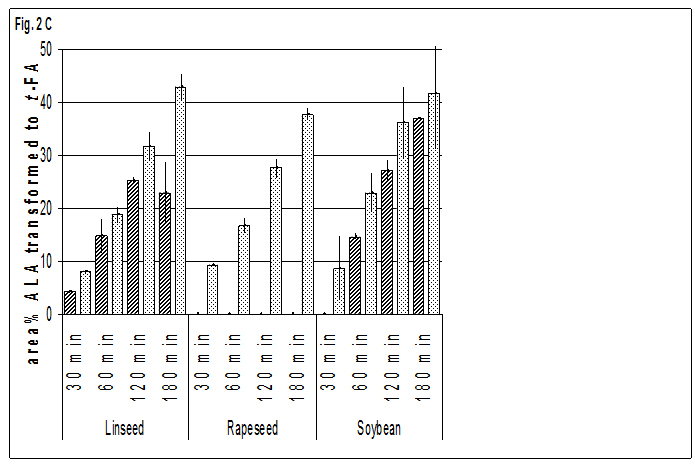 Fig. 2A-CFormation of trans-isomers=TI of oils
Fig. 2A-CFormation of trans-isomers=TI of oils and seeds
and seeds  oleic=OLA -, linoleic=LNA- and alpha-linolenic-acid=ALA in area % of all isomers at 523 K
oleic=OLA -, linoleic=LNA- and alpha-linolenic-acid=ALA in area % of all isomers at 523 K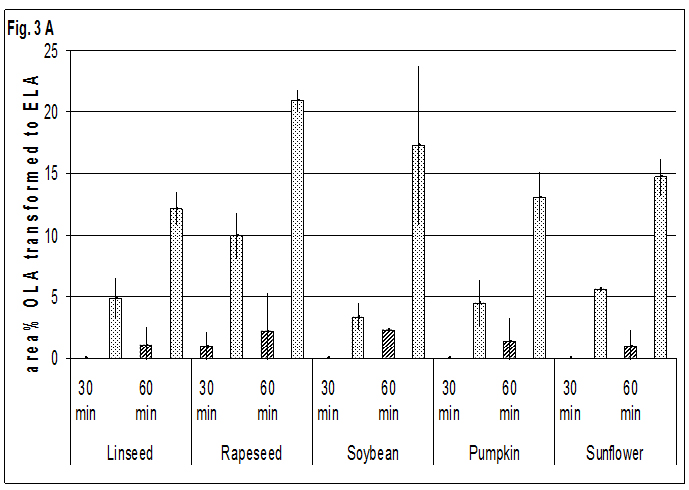
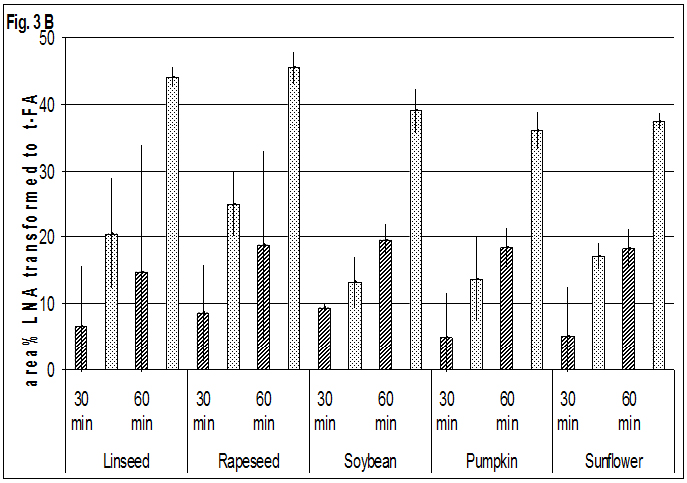
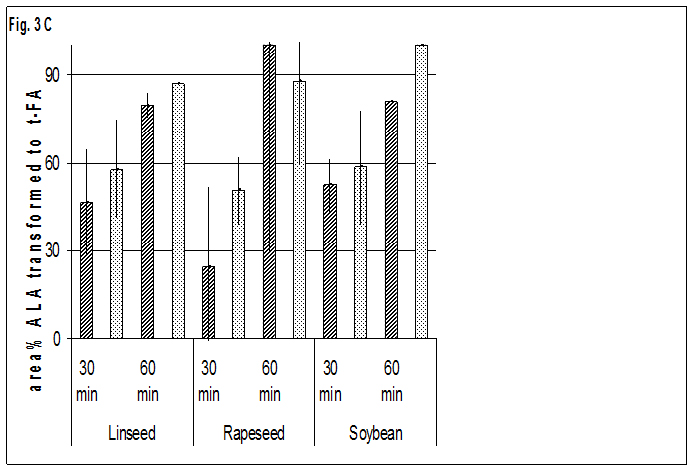 Fig. 3A-CFormation of trans-isomers=TI of oils
Fig. 3A-CFormation of trans-isomers=TI of oils  and seeds
and seeds oleic=OLA -, linoleic=LNA- and alpha-linolenic-acid=ALA in area % of all isomers at 573 K
oleic=OLA -, linoleic=LNA- and alpha-linolenic-acid=ALA in area % of all isomers at 573 K

