The influence of Bonsilage Plus and Bonsilage Forte on microflora reduction during ensiling of alfalfa
DOI:
UDK:
JOURNAL No:
Volume 37, Issue 2
PAGES
59-64
KEYWORDS
silage, alfalfa, lactic acid bacteria, inoculants
Ivana S. Čabarkapa*1, Dragan V. Palić1, Dragan B. Milić2, Miroslav V. Plavšić3, Dragana V. Plavšić1,
Đuro M. Vukmirović1, Radmilo R. Čolović1
1University of Novi Sad, Institute of Food Technology, 21000 Novi Sad, Bulevar cara Lazara 1, Serbia
2Institute of Field and Vegetable Crops, 21000 Novi Sad, Maksima Gorkog 30, Serbia
3University of Novi Sad, Faculty of Agriculture, 21000 Novi Sad, Trg D. Obradovića 8, Serbia
ABSTRACT
Abstract
Crops at ensiling contain both aerobic and anaerobic microorganisms and a range of bacteria and fungi that affect silage quality. Typical classes of microorganisms on plants prior to ensiling are aerobic bacteria, lactic acid bacteria (LAB), enterobacteria, yeasts, molds, clostridia, bacilli, acetic acid bacteria and propionic acid bacteria. The quality of silage depends on the competition between different groups of microorganisms. LAB, which are responsible for the silage fermentation process, usually dominate the silage microflora, but undesirable microorganisms, that occur at low levels on fresh plant material, may grow during the storage of silage and lead to anaerobic or aerobic spoilage. In this study, effects of silage inoculants on reduction of microflora during ensiling of alfalfa have been investigated. The results showed that the addition of commercial silage inoculants, Bonsilage Plus and Bonsilage Forte, had significant effect in reducing total number of aerobic bacteria, enterobacteria, yeasts, moulds and sulphite reducing clostridia during ensiling of alfalfa.
Introduction
Silage is an increasingly important source of animal feed in Serbia, where corn (Zea mays L), alfalfa (Medicago sativa L.) and sorghum (Sorghum bicolor L.) are popular silage crops widely used in ruminant nutrition.
Crops at ensiling contain both aerobic and anaerobic microorganisms and a range of bacteria and fungi that affect silage quality. Typical classes of microorganisms on plants prior to ensiling are aerobic bacteria, lactic acid bacteria (LAB), enterobacteria, yeasts, molds, clostridia, bacilli, acetic acid bacteria, and propionic acid bacteria (Pahlow et al., 2003).
Dominant populations are aerobic micro-organisms or facultative aerobes. Often LAB are lower in population than other groups of microorganisms on the crop at ensiling (Muck, 2010). The quality of silage depends on the competition between different groups of microorganisms. LAB, which are responsible for silage fermentation process, usually dominate the silage microflora (Dalié et al., 2010). In addition, a number of undesirable microorganisms that occur at low levels on fresh plant material may grow during the storage of silage and lead to anaerobic or aerobic spoilage (Giraffa et al., 2010).
Microbial silage inoculants containing lactic acid bacteria have long been used to improve silage fermentation. Inoculants were originally used to reduce pH and to avoid, or decrease, the risk of clostridial fermentation by the native bacterial population (Wilkinson et al., 2003).
The genus Lactobacillus belongs to the large group of LAB which are all gram-positive organisms and produce lactic acid by fermentation. There are two groups of species depending on the ability to ferment sugars: homo-fermentative species, converting sugars mostly into lactic acid, and hetero-fermentative species, converting sugars into lactic acid, acetic acid, ethanol and CO2 (Kandler and Weiss, 1986; Cabarkapa et al., 2010).
The heterofermentativesilage inoculants are aimed at controlling the development of aerobic spoilage microorganisms when the silage is exposed to air. These inoculants produce high levels of acetic acid that can suppress the growth of yeasts and molds. In most silage, yeasts are the initiators of aerobic deterioration because of their ability to grow relatively rapidly at low pH. Addition of inoculants has been effective in delaying aerobic microbial growth on silage, but it usually does not completely inhibit yeast growth (Muck, 2010).
Homofermentative inoculants are intended to minimize the activity of other microorganisms early in fermentation such as the enterobacteria and bacilli. If clostridial population is high at ensiling, these products could also reduce negative effects from clostridia during active lactic acid bacterial fermentation. Homofermentative inoculants, by reducing final pH, could have a role in preventing fermentation of lactic acid to butyric and clostridial development during storage. However, this effect would be expected in a limited number of cases – those where the natural fermentation fails in reducing the pH sufficiently to inhibit clostridia growth, but where further lowering of pH by added inoculant does inhibit them.
The aim of this study was to investigate effects of addition of two commercial silage inoculants on the reduction of microflora during ensiling of alfalfa.
MATERIALS AND METHODS
Fifth cut alfalfa, harvested in season 2010, was chopped at nominal particle length of approximately 20 mm. Material was manually compacted into laboratoryscale silos described by Čolovic et al. (2010). The alfalfa was treated in laboratory conditions with commercial silage inoculants Bonsilage Plus (BP)and Bonsilage forte (BF)(Schaumann, Austria) Bonsilage plus contains Pediococcus pentosaceus (DSM 12834), Lactobacillus brevis (DSM 12835), Lacto-bacillus buchneri (DSM 12856), Lacto-bacillus plantarum (DSM 12836) and Lacto-bacillus rhamnosus (NCIMB 30121). Other investigated inoculant Bonsilage forte contains combination of homofermentative strains with potent inhibitory action against clostridia Lactobacillus paracasei (DSM 16245), Lactobacillus lactis (NCIMB 30160), Pediococcus acidilactici (DSM 16243).
As a control was used alfalfa without added inoculant. Containers were divided in two groups. Each group consisted of three containers: first group with added Bonsilage Plus (BP), second with Bonsilage Forte (BF) and the third was a control group. The containers were stored at the temperature of 20 ± 3 C°. The sampling was conducted on days 7 and 55.
Total number of aerobic bacteria (TNB) was determined using the following procedure: 25 g of sample was transferre aseptically into individual stomacher bags containing 225 ml of sterile Saline Peptone Water (SPW) solution (0.1%) and homogenized in a stomacher for 60 seconds. For each sample, appropriate serial decimal dilution we prepared in the BPW solution. From each dilution step, 1.00 ml was transferred to a Petri dish. About 15 ml of agar tempered at 47 °C were poured into the Petri dish. Total number of all bacteria (except LAB) was determined aerobically using Plate Count Agar (PCA), after incubation for 3 days at 30 °C. LAB does not grow on this medium under aerobic conditions. Total number/count of yeasts (TNY) and Total number/count of moulds (TNM) was determined using Dichlor Rose Bengal Chloramphenicol agar (DRBC) after aerobic incubation at 25 °C for 5 days. Enterobacteriaceae were enumerated using Violet Red Bile Glucose agar (VRBG). For the VRBG, incubation was carried out at 37 °C for 24 h. Finally, sulphite reducing clostridia were enumerated using Tryptose-Sulfite agar (TS) agar after anaerobic incubation at 37 °C for 48 h. All plates were examined for typical colony types and morphology characteristics associated with each growth medium.
RESULTS AND DISCUSSION
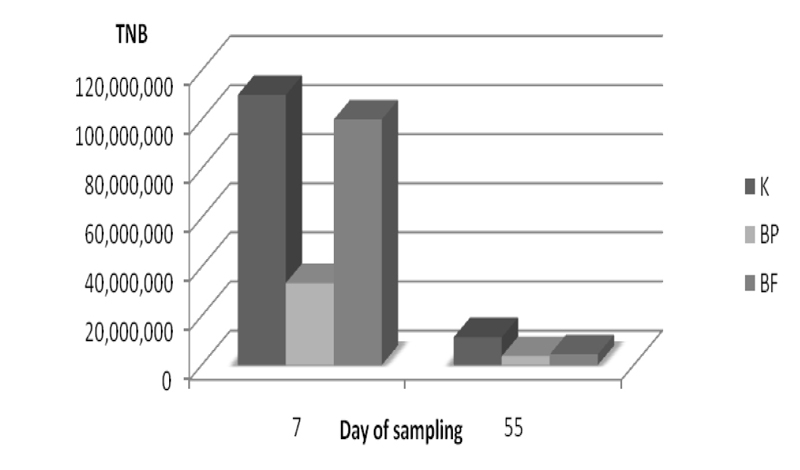
Reduction of the total number of aerobic bacteria in alfalfa silage with inoculants BP and BF on day 7 was 70% and 9.09% respectively, compared to the control sample.
On day 55 samples with inoculants BP and BF had reduction of 68.1% and 61.9% respectively compared to the control (Figure 1).
Reduction of the total number of entero-bacteria in silage with inoculants BP and BF on day 7 was 96.8% and 99.7% respectively compared to the control sample. On day 55 samples with inoculants BP and BF had reduction of 99.7% and 94.3% respectively compared to the control sample (Figure 2).
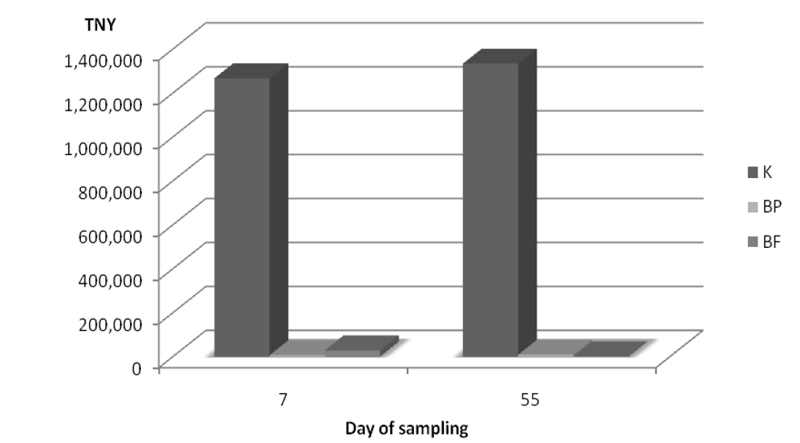
Reduction of the total number of yeasts in silage with inoculants BP and BF on day 7 was 99.5% and 97.7% respectively compared to the control sample on day 7. On day 55 samples with inoculants BP and BF had reduction of 99.3% and 99.9% respecttively compared to the control sample on day 55 (Figure 3).
According to Muck (2010) yeasts are the first group of microorganisms to develop once oxygen gets in contact with silage, either during storage or during feed out.
The reason for this is that many types of yeasts are capable of growing at pH 3.5, well below the pH of most silages.On day 7, total number of moulds in silage with inoculants BP and BF and in the control sample, was about the same, i.e. there was no moulds reduction.However, on day 55 samples with inoculants BP and BF had completed 100% reduction as compared to the control sample (Figure 4).
The volatile fatty acids are good inhibitors of yeasts and molds (Moon, 1983). The effect of acetic acid on fungal growth is related to the undissociated concentration in solution. Thus a given concentration of acetic acid becomes more inhibitory to yeasts and molds as silage pH decreases. Unfortunately, bacterial fermentation rarely lowers pH sufficiently and produces enough acetic acid to prevent yeasts and molds from growing in silage. Many yeasts and molds as well as acetic acid bacteria will grow at pH 3.5, well below normal silage pH. Once oxygen is present, yeasts, molds and acetic acid bacteria can begin to grow on silage, using fermentation products and residual sugars in the silage and producing carbon dioxide, water and heat. As fermentation products are used up, silage pH rises. Once the pH is above 4.5, a wide variety of other aerobic microorganisms can grow, spoiling the silage more and causing even higher heating of the silage. This causes losses, mainly of the most digestible parts of the silage. This spoilage loss can only be prevented by keeping oxygen out of the silo and minimizing silage expose to oxygen while emptying the silo (Muck, 2010).
Clostridia can grow at lower pH values than the enterobacteria making them more difficult to control. Reduction of the total number of sulphite reducing clostridia in silage with inoculants BP and BF on day 7 was 90% and 99% respectively compared to the control sample. On day 55 samples with inoculants BP and BF had reduction of 95.3% and 99.7% respectively compared to the control (Figure 5).
Ensiling preserves a crop by controlling microbial activity through a combination of an anaerobic environment and a natural fer mentation of sugars by LAB on the crop. Two fermentation products (lactic and acetic acids) and the resulting low pH primarily suppress the growth of other anaerobic microorganisms.
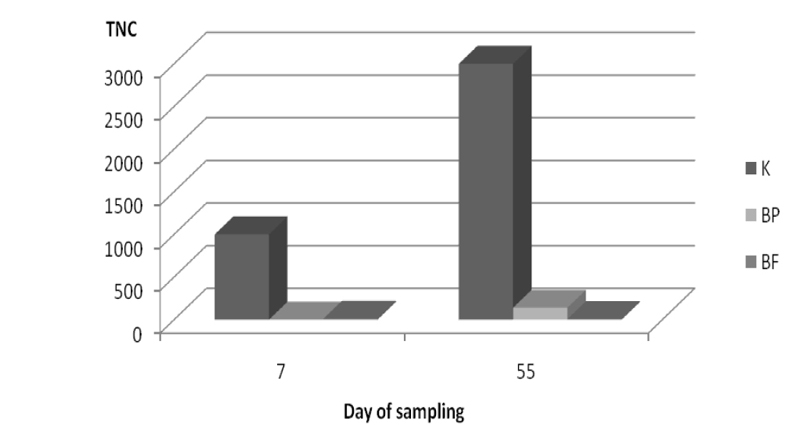 Figure 5. Reduction of total number of sulphite reducing clostridia (TNC) in alfalfa silage on days 7 and 55
Figure 5. Reduction of total number of sulphite reducing clostridia (TNC) in alfalfa silage on days 7 and 55The fermentation can also inhibit yeasts, molds and aerobic bacteria, but the anaerobic environment is essential to preventing most of the spoilage microorganisms from growing. There are two key processes that preserve the crop in the silo: the creation of an anaerobic environment and the fermentation of sugars by lactic acid bacteria to lactic acid and other products. Both processes are important for good preservation, and one can not substitute the other.
Conclusions
Good preservation and stabilization of the crop in the silo is due to the combination of an anaerobic environment and the growth of lactic acid bacteria (LAB). The LAB fermentation lower the pH and help to slow, but usually do not stop, the growth of aerobic spoilage microorganisms. Addition of silage inoculants promote the growth of LAB in an anaerobic environment and, by production of acetic acid and lowering of pH, prevent the growth of yeasts, molds and aerobic bacteria that spoil and heat the silage. The results of this study showed that the two commercial silage inoculants, Bonsilage Plus and Bonsilage Forte, had significant effect in reducing total number of aerobic bacteria, enterobacteria, yeasts, moulds and sulphite reducing clostridia during ensiling of alfalfa.
Acknowledgements
The authors are thankful to Schaumann (Agri-Austria, GmbH & Co. KG) for providing Bonsilage Plus and Bonsilage Forte.