THE INTRODUCTION OF A METHOD FOR DETERMINATION OF ORGANIC MATTER DIGESTIBILITY IN FEEDS INTO ROUTINE LABORATORY PRACTICE
DOI:
UDK:
JOURNAL No:
Volume 35, Issue 3
PAGES
151-154
KEYWORDS
Feeds, organic matter digestibility, in vitro method, accuracy, precision
Milica Pojić1*, Dragan Palić1, Jasna Mastilović1, Elizabet Janić-Hajnal1
1Institute for Food Technology, Novi Sad, Serbia
ABSTRACT
Abstract
The amount of available energy in feeds for ruminants can be described by organic matter digestibility (OMD), since the value of OMD is close to the corresponding digestibility of energy. In vivo trials with animals for determining OMD are expensive, time-consuming and not suited for routine analysis and there has been a constant search for alternative in vitro methods. The aim of this study was to introduce into routine laboratory practice an in vitro procedure for OMD estimation and to evaluate the possibility of its use for the development of near infrared spectroscopy (NIRS) calibration model for predicting OMD determined in vivo. Results of this study indicated existence of a certain bias, but it could not be considered as a significant spread since all obtained values were within the warning limits. Repeatability limit given to the in vitro method was fulfilled. Method showed such analytical characteristics that it can be utilized as the reference method for the development of NIRS prediction model.
INTRODUCTION
Energy value of ruminant feeds and its bio-availability is of great importance for animal feed manufactures and end users. The amount of available energy in feeds for ruminants is described either by its metabolisable energy or by organic matter digestibility (Barber et al., 1990), since the value of organic matter digestibility is very close to the corresponding digestibility of energy (Thomas, 1990). The most accurate way of obtaining information on digestibility of organic matter of feeds for ruminants is by conducting in vivo digestibility experiments. Since this method is expensive, time-consuming and therefore is not suited for routine analysis, there has been a constant search for laboratory methods for routine prediction of the in vivo organic matter digestibility of ruminant feeds, in order to implement an adequate system of quality control in the feed industry.
In vitro methods used to predict the in vivo digestibility of organic matter of feeds for ruminants are generally based on: chemical analysis, fermentation with rumen micro-organisms and hydrolysis with enzymatic preparations.
Prediction of organic matter digestibility of forages using chemical analysis by Weende method or Acid detergent fibre method resulted in variable correlations (Aufrère & Michalet-Doreau, 1988).
Methods for predicting in vivo organic matter digestibility using rumen liquor fermentation techniques have become well established, but there are limitations on the use of rumen liquor for digestibility studies. There must be fistulated animals (which are not available to all laboratories) for the collection of fresh rumen liquor. There is also a concern over the use of surgically modified animals in experimentation (Jones & Theodorou, 2000).
The use of incubation of feeds with exogenous enzymes to predict the in vivo organic matter digestibility has the aim to mimic the digestive process in the animal. Most enzymatic methods for organic matter digestibility estimation have been developed for forage feedstuffs, with a few used for other feedstuffs, e.g. grains and compound feeds (Aufrère & Michalet-Doreau, 1988). Weisbjerg and Hvelplund (1993) developed a multi-enzymatic incubation method for estimation of enzymatic digestibility of organic matter for use on compound feeds. This procedure also showed an ability to estimate the organic matter digestibility of straws (Hvelplund, Weisbjerg & Søegaard, 1999), which demonstrated the potential of this method to predict the in vivo organic matter digestibility of grains and forages. Apart from the above-mentioned two references, more citations of this method the literature have not been found.
The aim of this study was to introduce this under-exploited in vitro enzymatic method for the determination of organic matter digestibility (OMD) of feed in the laboratory practise and to determine some of the method characteristics (accuracy and precision), in order to evaluate the possibility of its use for the development of near infrared spectroscopy calibration model for prediction of the in vivo OMD.
MATERIALS AND METHODS
The in vitro method of Weisbjerg and Hvelplund (1993) used in this study consists of several successive steps: treating of dried and grounded sample with pepsin-hydrochloric acid to dissolve the protein content, hydrolysis of starch and inactivation of pepsin at 80 °C, incubation the sample with the mixture of enzymes to dissolve digestible carbohydrates from cell walls, extraction of fat with boiling water and acetone and determination of dry matter content and ash of the undissolved residue.
Materials
The set of five reference samples with the known values of organic matter digestibility (OMD) were used. They comprises of different types of feed such as concentrate mix, palm kernel cake, sunflower meal, barley and straw, with the range of OMD values from 35,3 to 91,7%. The reference values were determined by the Department of Animal Health, Welfare and Nutrition (Faculty of Agricultural Sciences, University of Aarhus), Tjele, Denmark.
Methods
0.25 g of grounded sample with 1 mg accuracy was measured in a 30-ml filter crucible porosity 2 and 15 ml of pepsinhydrochloric acid solution was added. The crucibles were closed with rubber plugs and incubated for 24 hours at 40 °C. Upon the expiration of this period the crucibles were incubated at 80°C for 45 minutes. The plugs from the crucibles were removed and the liquid was sucked away. The content of the crucibles were washed with 100 ml of water. Fifteen ml of buffered enzyme solution, consisting of Amyloglukosidase (activity 3260 Uml-1) and commercial enzymes: Celluclast 1.5 L FG (activity 798 EGU g-1), Novozym 51054 (activity 1000 KVHCU(m) g-1), Vyscozyme L (activity 120 FBG ml-1, was added to the content of the crucibles. They were firstly incubated at 40°C for 24 hours, and then at 60 °C for 19 hours. Upon the expiration of this period the liquid from the crucibles were sucked away, and the content of the crucibles were firstly washed with 100 ml of boiling water, and then with 20 ml of acetone. The crucibles with the content were dryed at 103°C until a constant weight was reached. After drying the crucibles were cooled down in the desiccator and immediately weighted. The content of the crucibles were then incinerated at 475-500 oC until a constant weight is achieved. A blank correction without matter was included. In order to calculate the OMD, the crude ash content of the samples was determined by incineration at 500 oC.
It is to be pointed out that the original in vitro OMD method (Weisbjerg and Hvelplund, 1993) used 100-ml filter crucible, 0.5 g of sample and 30 ml of enzyme buffer solution, whereas in this study 30-ml crucibles were used, which required decreasing of sample mass to 0.25 g and enzyme buffer volume to 15 ml.
The samples were analysed in ten independent incubations and in blind duplicates.
The content of organic matter (OM) as a percentage of the sample was calculated by the formula:
OM= 100 - Crude ash (%)The insoluble content of organic matter as a percentage of the sample was calculated by the formula:
Insoluble OM= (b − c) ∗100 a
where a is gram weighted out sample, b is weight loss during incineration of the precipitate from the sampling at 103 ºC, and c is weight loss during incineration of the precipitate from the blank specimen at 103 ºC.
The organic matter digestibility (OMD) was calculated by the formula:
OMD = (OM- Insoluble OM)* 100OM
Statistical evaluation
For the monitoring of the validity of tests undertaken, X and R control charts were plotted. Since the reference samples were used, the central lines of X charts were set to the nominal value, while the set of control limits were based on the requirement on the analytical quality. The standard deviation for the X control chart (s) was estimated from the requirement on standard deviation of reproducibility (sR), so the warning limits were set at ± 2s, while the action limits were set at ± 3s. Concerning R control chart, the set of control limits were based on the requirement on repeatability (standard deviation of repeatability – sr). Since the measurement was carried out in replicate (n=2) the central line was estimated as 1,128*s, upper warning limit as 2,83*s, and upper action limit as 3,69*s (Hovind et al., 2007, ISO 8258).
RESULTS AND DISCUSSION
Accuracy
The results obtained by the OMD determination in the selected reference samples are shown in the figures 1-5.
This approach of monitoring newly introduced analytical procedure was chosen due to the fact that it involves a continuous and critical evaluation of the laboratory’s analytical methods, from the sample preparation to the final analytical report. It was chosen to monitor the systematic and random effects on the method results based on the results of the reference samples.
The OMD values for concentrate mix, palm kernel cake, sunflower meal, barley and straw were 91,7; 65,5; 72,3; 91,1, and 35,3, respectively, while the obtained results were in the range 87,5-90,6%; 65,0-66,7%; 74,0-75,7%; 89,4-91,0%; and 34,1-36,0%, respectively. The determinations of OMD in palm kernel cake (Fig. 2) and in straw (Fig. 5) were in control since the control values were within the warning limits. The determinations of OMD in concentrate mix (Fig. 1), sunflower meal (Fig. 3) and barley (Fig. 4) were in control but can be regarded as out of statistical control, because all the control values were within the warning limits and were lying on the same side of the central line. In this case, the results tended to be always greater or smaller than the reference values, indicating that the bias has taken place. The bias refers to the systematic errors in analytical procedure and is defined as the difference between the accepted reference value and the mean value of a great number of analytical results. Due to its nature it is used in terms of the trueness of the analytical method.
The possible sources of systematic error that resulted with bias could be caused by the time span between the initial sample analysis at the Department of Animal Health, Welfare and Nutrition, Tjele, Denmark, as well as by the decrease in the sample mass and enzyme buffer volume.
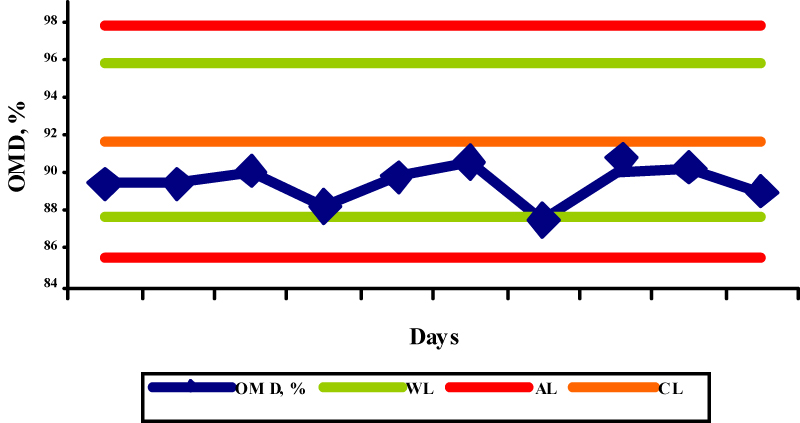 Fig.1. X control chart for the determination of OMD in concentrate mix (WL-warning limits; AL-action limits; CL-central line)
Fig.1. X control chart for the determination of OMD in concentrate mix (WL-warning limits; AL-action limits; CL-central line)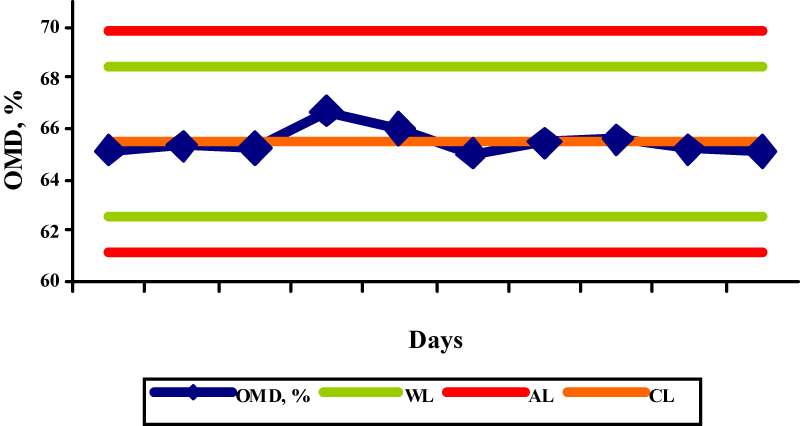 Fig.2. X control chart for the determination of OMD in palm kernel cake (WL-warning limits; AL-action limits; CL-central line)
Fig.2. X control chart for the determination of OMD in palm kernel cake (WL-warning limits; AL-action limits; CL-central line)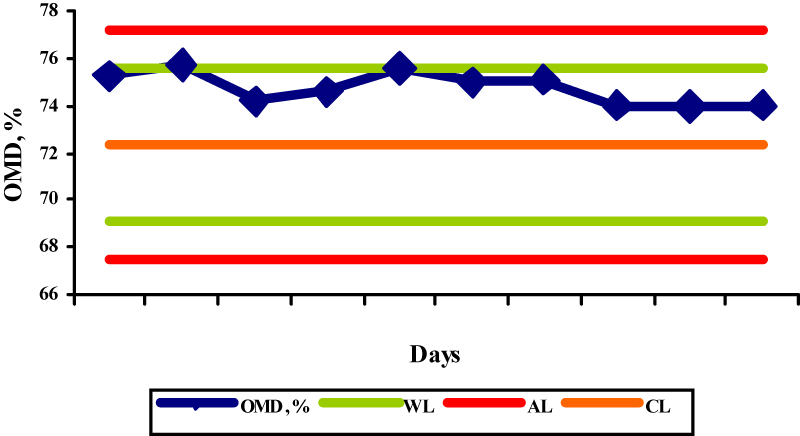 Fig.3. X control chart for the determination of OMD in sunflower meal (WL-warning limits; AL-action limits; CL-central line)
Fig.3. X control chart for the determination of OMD in sunflower meal (WL-warning limits; AL-action limits; CL-central line)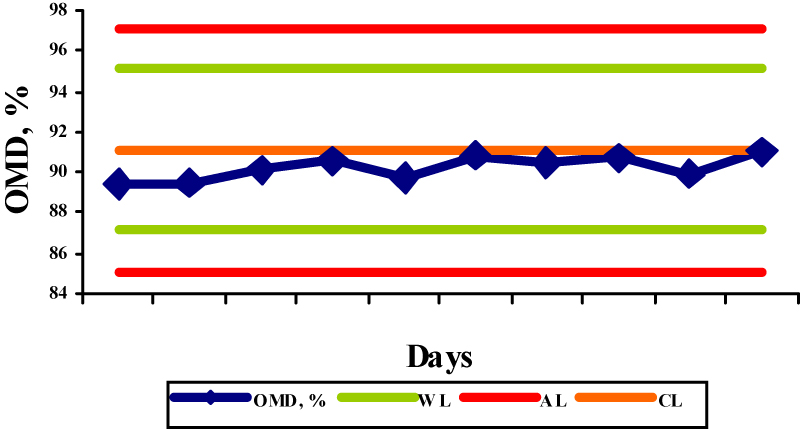 Fig.4. X control chart for the determination of OMD in barley (WL-warning limits; AL-action limits; CL-central line)
Fig.4. X control chart for the determination of OMD in barley (WL-warning limits; AL-action limits; CL-central line)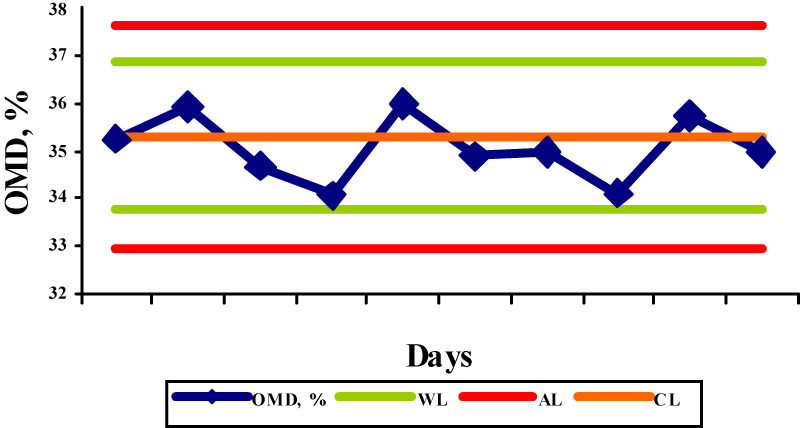 Fig.5. X control chart for the determination of OMD in straw (WL-warning limits; AL-action limits; CL-central line)
Fig.5. X control chart for the determination of OMD in straw (WL-warning limits; AL-action limits; CL-central line)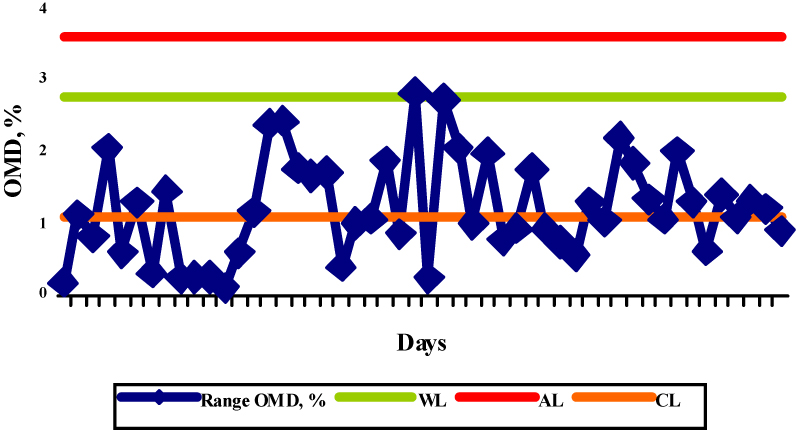 Fig.6. R control chart for the determination of OMD in the reference samples (WL-warning limit; AL-action limit; CL-central line)
Fig.6. R control chart for the determination of OMD in the reference samples (WL-warning limit; AL-action limit; CL-central line)Precision
In order to test the repeatability of a newly introduced method the range chart was plotted (Fig. 6), where the range representted difference between the values obtained in duplicate determination. The control limits were set up according to the analytical requirement – target control limits. The target control limits were calculated from a standard deviation, i.e. a target for repeatability of the method used. All values plotted in the R chart did not exceed the action limit, whereas the two values were on the warning limit indicating that the repeatability limit given in the method was fulfilled.
CONCLUSIONS
The obtained results showed that the certain bias was noticed, but it could not be considered as significant spread of the results since all obtained results were within the warning limits. These results were out of statistical control, but might have been acceptable. In order to draw more reliable-conclusion, the bias should be monitored for a longer time period. Furthermore, the repeatability limit given in the method was fulfilled, so the method showed such the analytical characteristics that it can be uti-lize as the reference method for the de-velopment of NIRS prediction model.
ACKNOWLEDGEMENTS
This work was financed by the Ministry of Science and Technological Development, Republic of Serbia (Project No 20066). We also thank University of Aarhus, Faculty of Agricultural Sciences, Department of Ani-mal Health, Welfare and Nutrition, Tjele, Denmark especially to Dr. Martin Weisbjerg for his assistance in the acquisition of enzy-mes and reference samples.


